Question: For the metal iron (Fe), with parameters as follows, plot the Lennard Jones potential in DESMOS and obtain: The average distance between the atoms

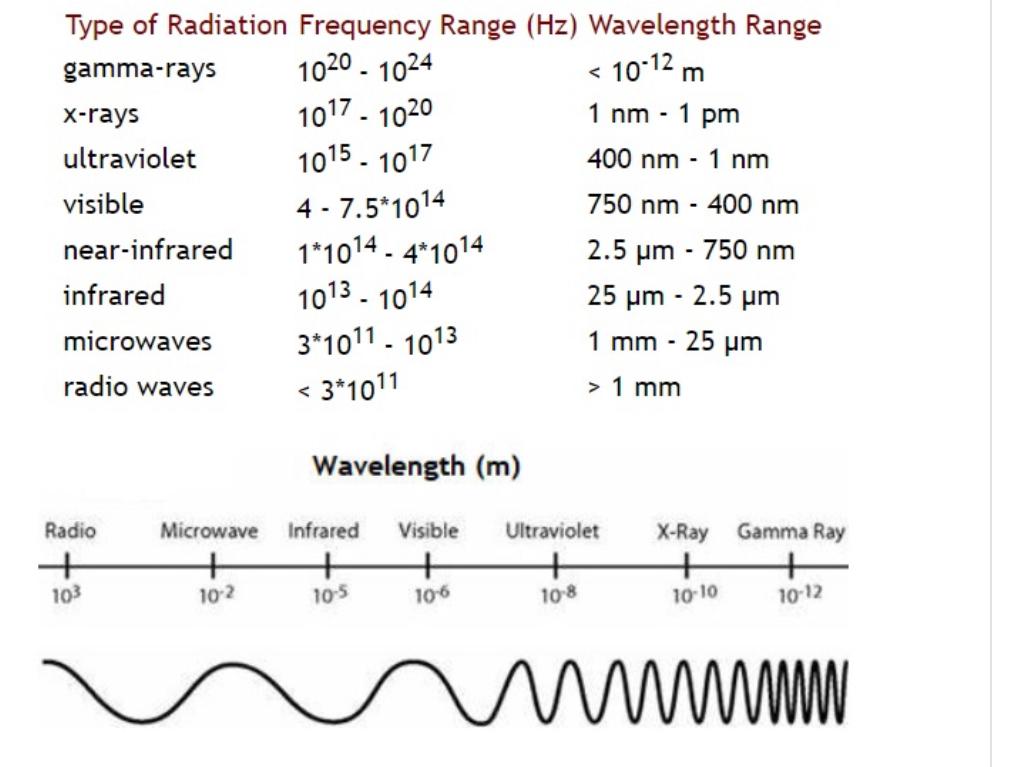
For the metal iron (Fe), with parameters as follows, plot the Lennard Jones potential in DESMOS and obtain: The average distance between the atoms when the energy is minimum (the x-axis is in Angstrom, where 1 A = 1*10-10m). Answer TO The energy needed to break the bond (the y-axis is in electron volts, where leV = 1.60218*10-19J). Answer eV The average distance between the atoms if the total energy (the one above the minimum) were half the energy needed to break the bond. (You can estimate that this distance falls in the middle between the two extremes of the vibration movement with that amount of energy.) Answer The wavelength of a photon with enough energy to break the bond (NOTE: this is MORE than just moving to the next vibrational energy level). The energy of a photon is E hc/1 where his Plank's constant, c is the speed of light in vacuum, and I is the wavelength of the color of light to which that photon corresponds. Inm=1* 10-9m Answer nm Identify whether the photon is from a radio wave, microwave, infrared light, visible light, ultraviolet light, X-rays, or gamma rays. Answer Lennard-Jones potential parameters Fe = 2.4193 and = 0.2007 eV melting point TL = 1760.8 K discrepancy 2.85% Ni = 1.5808 and & = 0.1729 eV melting point TL = 1726.8 K discrepancy 0.07% Pb = 3.6888 and = 0.0610 eV melting point TL = 598.7 K discrepancy of 0.32% Cr = 2.7813 and &= 0.24322 eV melting point TL = 2183.7 K discrepancy 0.17%. gamma-rays Type of Radiation Frequency Range (Hz) Wavelength Range 1020-1024 < 10-12 m x-rays 1017-1020 1 nm - 1 pm ultraviolet 1015 1017 400 nm - 1 nm visible 4-7.5*1014 750 nm - 400 nm near-infrared 1*1014 - 4*1014 2.5 m - 750 nm infrared microwaves radio waves 10131014 25 km - 2.5 um 3*1011 1013 1 mm - 25 m < 3*1011 > 1 mm Wavelength (m) Radio Microwave Infrared Visible Ultraviolet X-Ray Gamma Ray + 103 + 10-2 + + + + 10-5 10-6 10-8 10-10 + 10-12 wwwwwww
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


