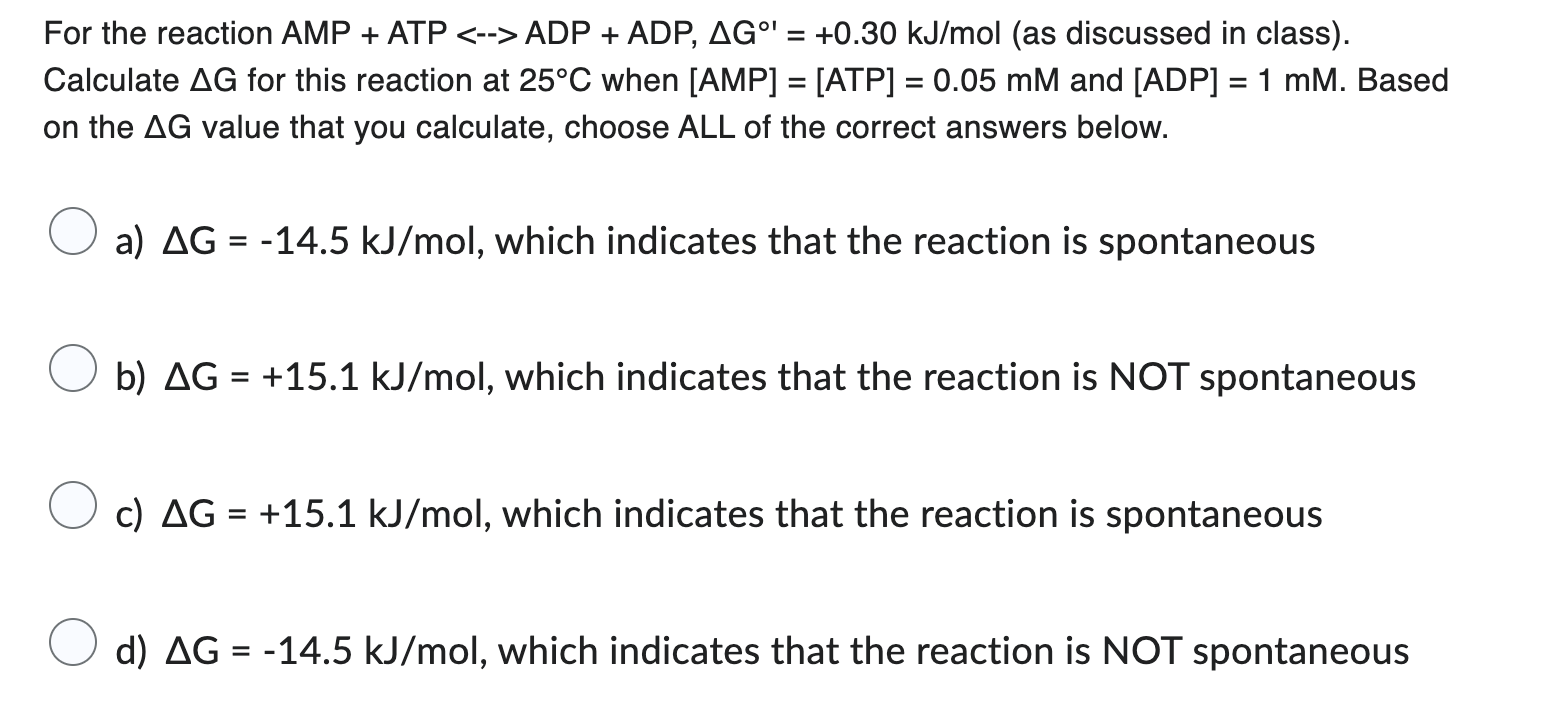
Question: For the reaction AMP + ATP - ADP + ADP, G @ ' = + 0 . 3 0 k J m o l (
For the reaction AMP ATP ADPADP,as discussed in class
Calculate for this reaction at when and Based
on the value that you calculate, choose ALL of the correct answers below.
a which indicates that the reaction is spontaneous
b which indicates that the reaction is NOT spontaneous
c which indicates that the reaction is spontaneous
d which indicates that the reaction is NOT spontaneous
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


