Question: part 1 = -60.7 (correct) part 2 = (incorrect) not .98 or .99 part 3 = (correct) The adenylate system manages the short-term eneriy needs
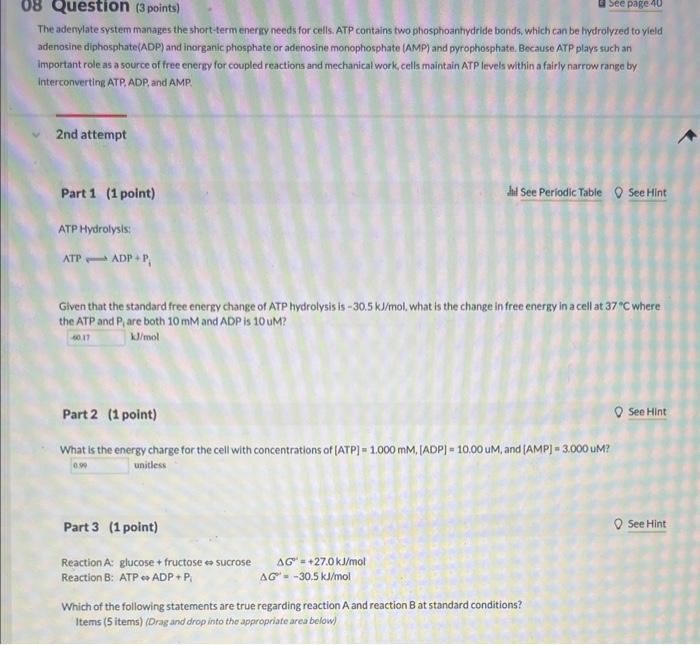
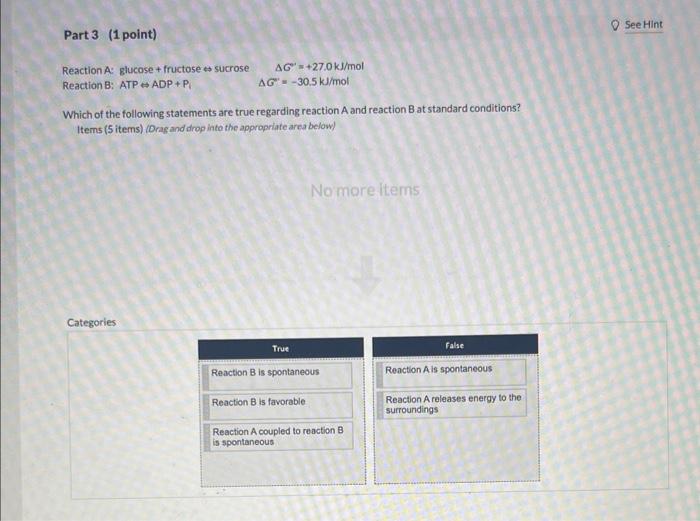
The adenylate system manages the short-term eneriy needs for cells. ATP contains two phosphoantiydride bonds, which can be hydrolyzed to yield adenosine diphosphate(ADP) and inorganic phosphate or adenosine monophosphate (AMP) and pyrophosphate. Bccause ATP plays such an important role as a source of free energy for coupled reactions and mechanical work, cells maintain ATP levels within a fairly narrow range by interconverting ATP, ADP, and AMP. 2nd attempt Part 1 (1 point) hal see PeriodicTable 0 SeeHint ATP Hydrolysis: ATPADP+Pi Given that the standard free energy change of ATP hydrolysis is 30.5kJ/mol, what is the change in free energy in a cell at 377C where the ATP and Pi are both 10mM and ADP is 10 uM? kJ/mol Part 2 (1 point) See Hint What is the energy charge for the cell with concentrations of [ATP]=1.000mM,[ADP]=10.00 uM, and [AMP]=3.000 uM? unitess Part 3 (1 point) See Hint Reaction A: glucose + fructose sucrose G=+27.0kJ/mol Reaction B: ATP ADP +Pi=30.5kJ/mol Which of the following statements are true regarding reaction A and reaction B at 5 tandard conditions? Items (5 items) (Drag and drop into the appropriate area below) Reaction A: glucose + fructose sucrose G=+27.0kJ/mol Reaction B: ATP ADP+P =30.5kJ/mol Which of the following statements are true regarding reaction A and reaction B at standard conditions? Items (5 items) (Drag and drop into the appropriate area below)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


