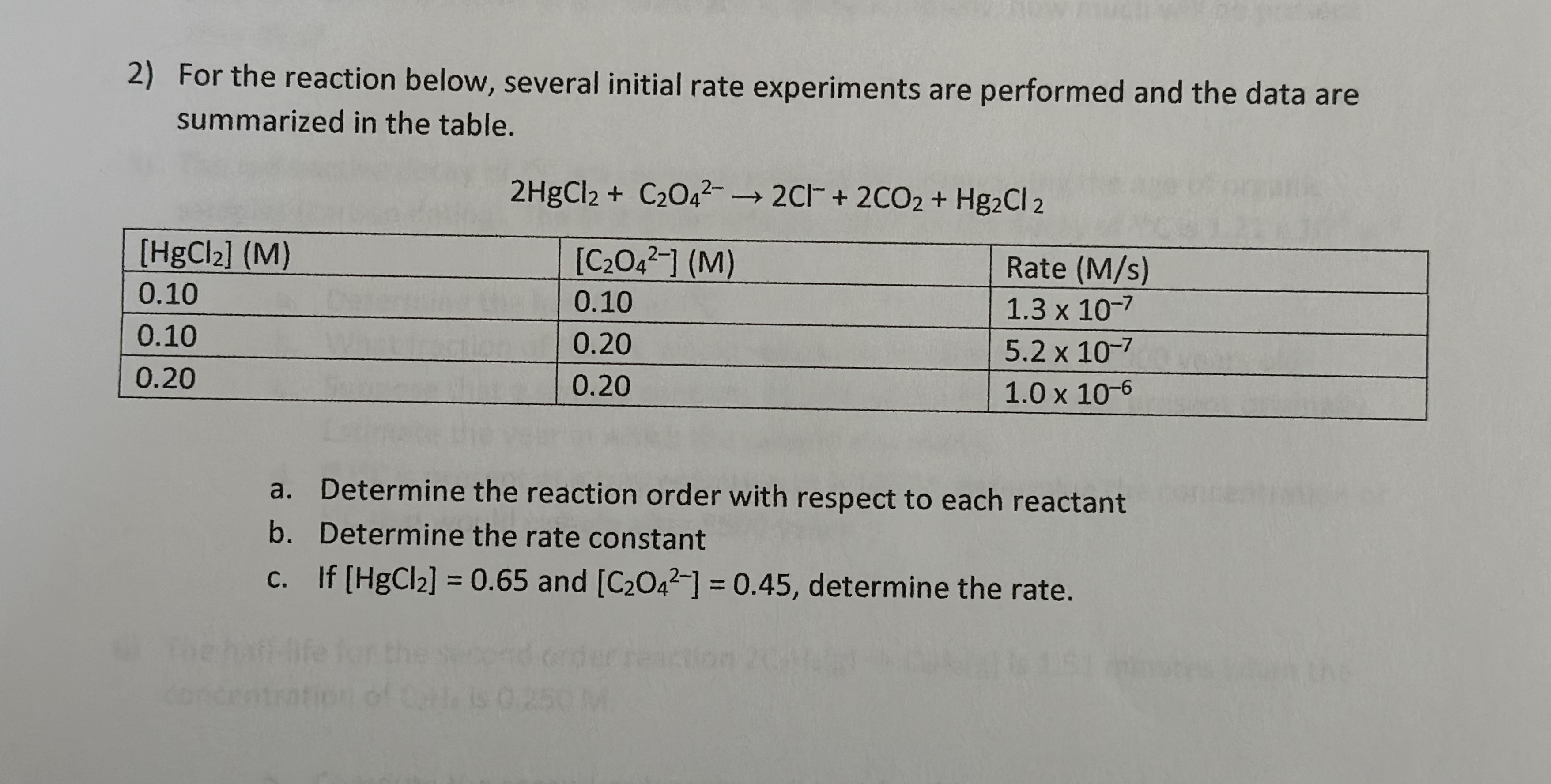
Question: For the reaction below, several initial rate experiments are performed and the data are summarized in the table. 2 H g C l 2 +
For the reaction below, several initial rate experiments are performed and the data are
summarized in the table.
a Determine the reaction order with respect to each reactant
b Determine the rate constant
c If and determine the rate.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


