Question: For the reaction below, several initial rate experiments are performed and the data are summarized in the table. 2HgCl2+C2O422Cl+2CO2+Hg2Cl2 a. Determine the reaction order with
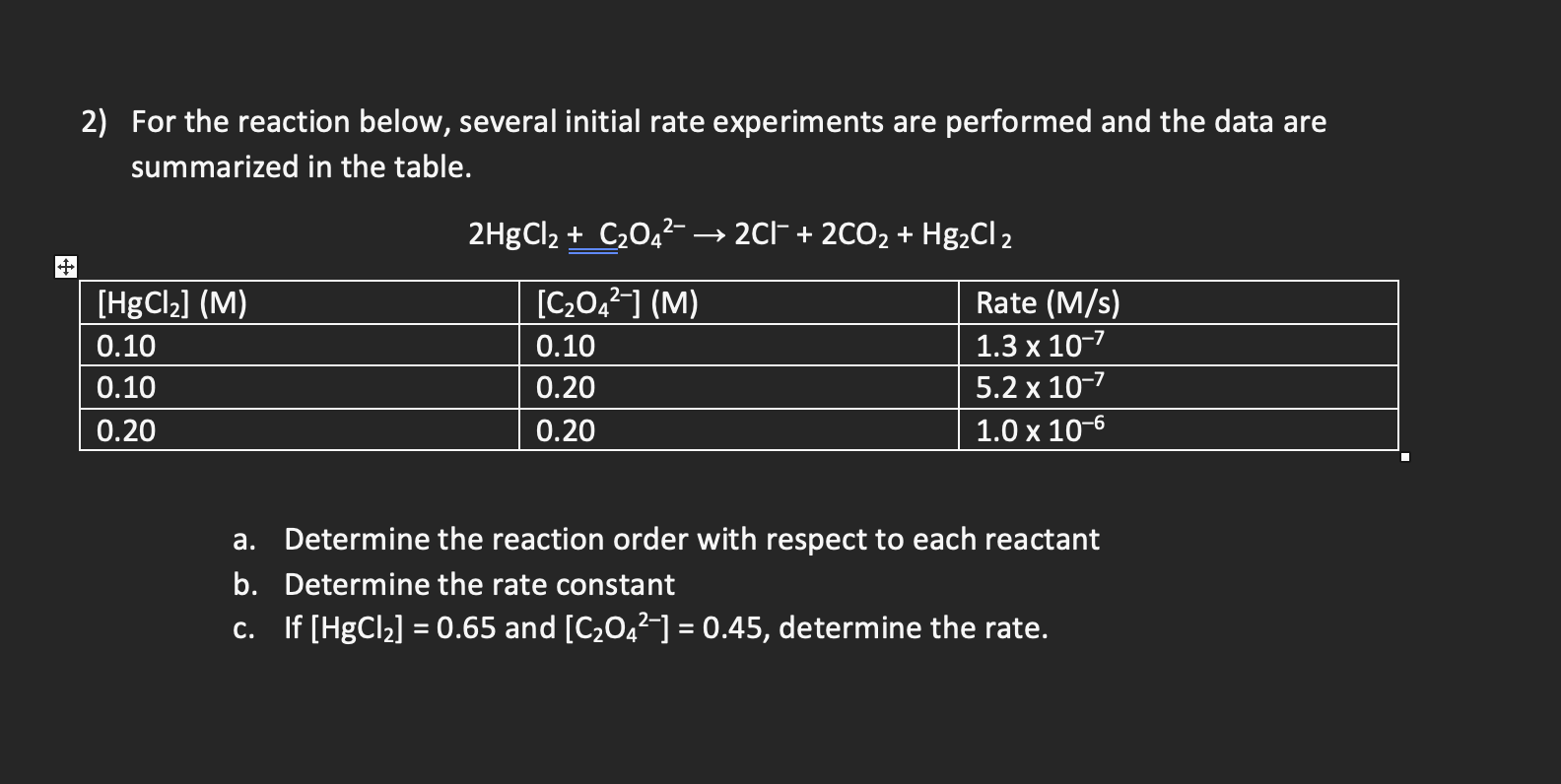
For the reaction below, several initial rate experiments are performed and the data are summarized in the table. 2HgCl2+C2O422Cl+2CO2+Hg2Cl2 a. Determine the reaction order with respect to each reactant b. Determine the rate constant c. If [HgCl2]=0.65 and [C2O42]=0.45, determine the rate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


