Question: For the reaction scheme below, tabel each reactant as either a Lewis acid/base (LA or LB) of a Bronsted Lowry acid/bsie (BLA or BL B).
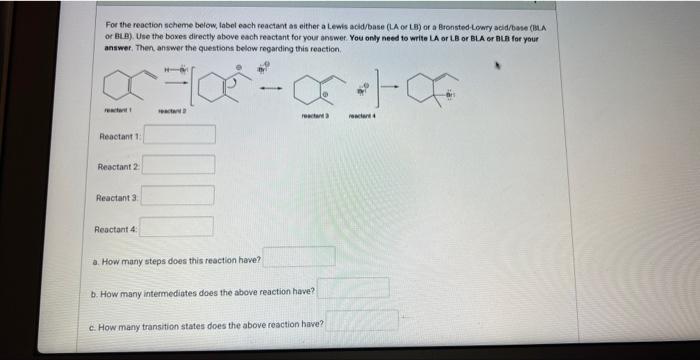
For the reaction scheme below, tabel each reactant as either a Lewis acid/base (LA or LB) of a Bronsted Lowry acid/bsie (BLA or BL B). Use the boxes directly above each resctant for your answer. You only need to write LA or LB or BLA or ati for your answer, Then answer the questions below regarding this reaction. Peactant 1 : Reoctant 2 : Reactant 3. Peactant 4: a. How many steps does this reaction have? b. How many intermediates does the above reaction have? c. How many transition states does the above reaction have
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


