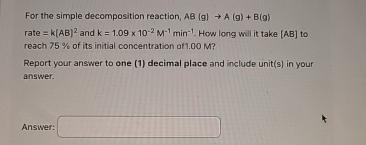
Question: For the simple decomposition reaction, A B ( g ) A ( g ) + B ( g ) rate = k ( A B
For the simple decomposition reaction, rate and How long wil it take to reach of its initial concentration at
Report your answer to one decimal place and include units in your answer.
Answe
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


