Question: For the solution below, determine the pH at equilibrium if 0.15 mmol/L of HCl is added. A buffered water to which a combination of H
For the solution below, determine the pH at equilibrium if 0.15 mmol/L of HCl is added.
A buffered water to which a combination of H2CO3 and NaCO3 has been added, which has a total carbonate concentration (CT) of 100 mmol/L and a pH of 7. Assume [CO32] is negligible at all times, no exchange of carbonate/CO2 with the atmosphere occurs (CT is constant), and the following reactions are relevant:
H2CO3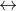 HCO3 + H+ pKA = 6.3
HCO3 + H+ pKA = 6.3
Water dissociation pKw = 14
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


