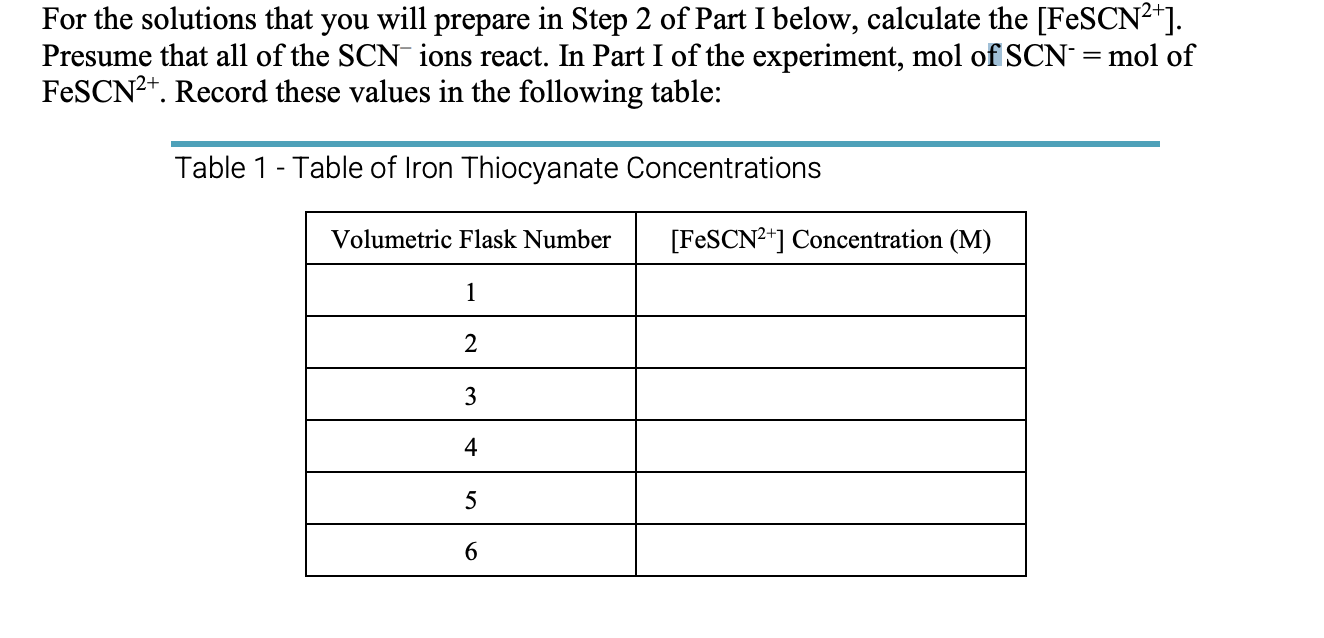
Question: For the solutions that you will prepare in Step 2 of Part I below, calculate the [FeSCN2+]. Presume that all of the SCNions react. In
![Part I below, calculate the [FeSCN2+]. Presume that all of the SCNions](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f70a7ed9204_11066f70a7e78121.jpg)
For the solutions that you will prepare in Step 2 of Part I below, calculate the [FeSCN2+]. Presume that all of the SCNions react. In Part I of the experiment, mol of SCN=mol of FeSCN2+. Record these values in the following table: Table 1 - Table of Iron Thiocyanate Concentrations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


