Question: please answer these questions below: har 2. Label four 20 x 150 mm test tubes 1-4. Pour about 30 mL of 0.0020 M Fe(NO3)3 into
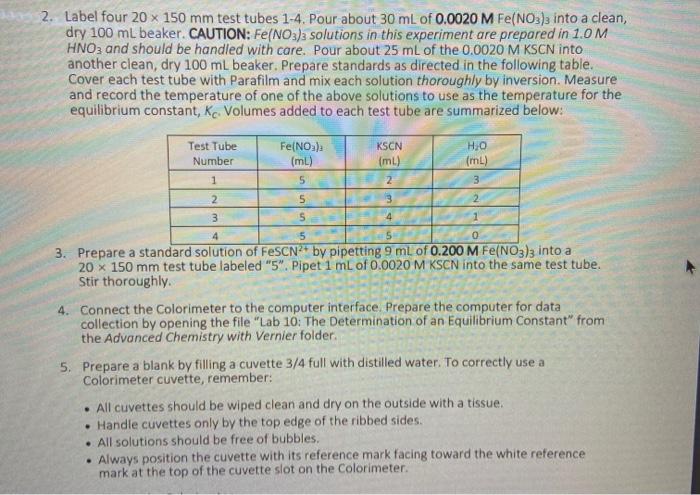



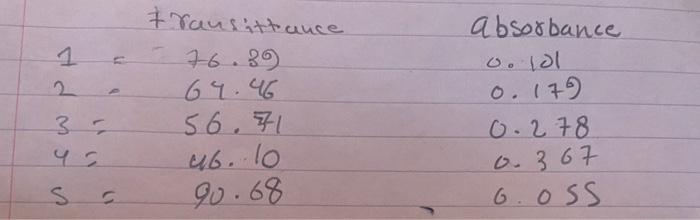
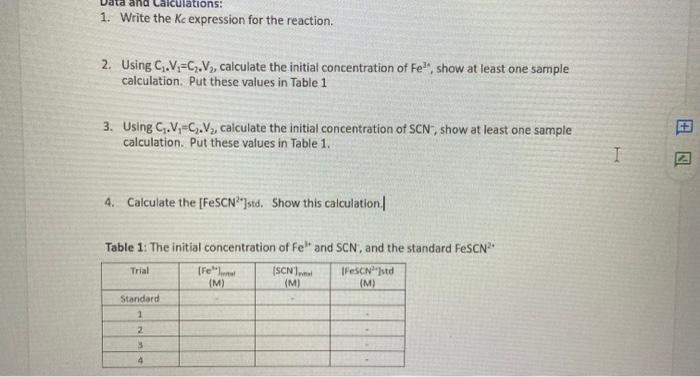
har 2. Label four 20 x 150 mm test tubes 1-4. Pour about 30 mL of 0.0020 M Fe(NO3)3 into a clean, dry 100 ml beaker. CAUTION: Fe(NO3)2 solutions in this experiment are prepared in 1.0 M HNO3 and should be handled with care. Pour about 25 mL of the 0.0020 M KSCN into another clean, dry 100 ml beaker. Prepare standards as directed in the following table. Cover each test tube with Parafilm and mix each solution thoroughly by inversion. Measure and record the temperature of one of the above solutions to use as the temperature for the equilibrium constant, Kc. Volumes added to each test tube are summarized below: Test Tube Number 1 Fe(NO) (ml) 5 5 KSCN (ml) 2 3 4 HO (mL) 3 2 2 3 5 4 3. Prepare a standard solution of FeSCN? by pipetting 9 ml of 0.200 M Fe(NO3)3 into a 20 x 150 mm test tube labeled "5". Pipet 1 mL of 0.0020 M KSCN into the same test tube. Stir thoroughly. 4. Connect the Colorimeter to the computer interface. Prepare the computer for data collection by opening the file "Lab 10: The Determination of an Equilibrium Constant" from the Advanced Chemistry with Vernier folder 5. Prepare a blank by filling a cuvette 3/4 full with distilled water. To correctly use a Colorimeter cuvette, remember: All cuvettes should be wiped clean and dry on the outside with a tissue Handle cuvettes only by the top edge of the ribbed sides. All solutions should be free of bubbles. Always position the cuvette with its reference mark facing toward the white reference mark at the top of the cuvette slot on the Colorimeter. . 6. Cambrate the colorimeter a. Open the Colorimeter lid. b. Holding the cuvette by the upper edges, place it in the cuvette slot of the Colorimeter. Close the lid. c. If your Colorimeter has a CAL button, Press the cor > button on the Colorimeter to select a wavelength of 470 nm (Blue) for this experiment. Press the cal button until the red LED begins to flash. Then release the CAL button. When the LED stops flashing, the calibration is complete. Proceed directly to Step 7. If your Colorimeter does not have a CAL button, continue with this step to calibrate your Colorimeter. First Calibration Point d. Choose Calibrate CH1: Colorimeter (%T) from the Experiment menu and then click e. Turn the wavelength knob on the Colorimeter to the "%T" position. f. Type "O" in the edit box 8. When the displayed voltage reading for Reading 1 stabilizes, click Keep 75 Calibrate Now cond Calibration Point h. Turn the knob of the Colorimeter to the Blue LED position (470 nm). 1. Type "100" in the edit box. 1. When the displayed voltage reading for Reading 2 stabilizes, click Keep then click Done Second Calibration Point h. Turn the knob of the Colorimeter to the Blue LED position (470 nm). i. Type "100" in the edit box. j. When the displayed voltage reading for Reading 2 stabilizes, click Keep then click Done 7. You are now ready to collect absorbance data for the four equilibrium systems and the standard solution a. Click Collect to begin data collection b. Empty the water from the cuvette. Rinse it twice with 1 mL portions of the Test Tube 1 solution. c. Wipe the outside of the cuvette with a tissue and then place the cuvette in the Colorimeter. After closing the lid, wait for the absorbance value displayed in the meter to stabilize. Then click keep type "1" (the trial number) in edit box, and press the ENTER key. d. Discard the cuvette contents as directed by your teacher. Rinse the cuvette twice with the Test Tube 2 solution and fill the cuvette 3/4 full. Follow the Step-c procedure to find the absorbance of this solution. Type "2" in the edit box and press ENTER. e. Repeat the Step-d procedure to find the absorbance of the solutions in Test Tubes 3, 4, and 5 (the standard solution). f. From the table, record the absorbance values for each of the five trials in your data table. 8. Dispose of all solutions as directed by your instructor PROCESSING THE DATA 1. Write the Kc expression for the reaction in the Data and Calculation table. 2. Using C1.V=C2V2, calculate the initial concentration of Fe, based on the dilution that results from adding KSCN solution and water to the original 0.0020 M Fe(NO3)3 solution. See Step 2 of the procedure for the volume of each substance used in Trials 1-4. This should be the same for all four test tubes. 3. Using C1.V1=C2.V2, calculate the initial concentration of SCN", based on its dilution by Fe(NO3)3 and water. This will differ for each trial. 4. Calculate the (FeSCN?*]std. Recall that a high initial concentration of Fe3+ ions forces the reaction far to the right, using up nearly 100% of the initial SCN-ions. Using stoichiometry and the balanced equation, it is assumed that for every mole of FeSCN2+ produced, one mole of SCN is used up. So it is assumed that (FeSCN2+Istd, is equal to the [SCN-)Using C1.V =C2.V2, calculate the initial concentration of SCN from step 3 based on its dilution with Fe(NO3)3. 1 IdM transittance 76.39 64.46 56.71 absorbance o. lol 0.179 0.278 3 - 0.367 ub. lo 90.68 S c S 6.OSS alculations: 1. Write the Kc expression for the reaction. 2. Using C.V=C.V, calculate the initial concentration of Fe', show at least one sample calculation. Put these values in Table 1 3. Using C.-C. Vz, calculate the initial concentration of SCN, show at least one sample calculation. Put these values in Table 1. I 4. Calculate the [FeSCN"std. Show this calculation | Few Table 1: The initial concentration of Fe and SCN, and the standard FeSCN Trial (SCN1... Festd (M) (M) (M) Standard 1 2 3 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


