Question: For this reaction: Melting points are 2 Na + Cl2 2 NaCl 100 C -100 C 800 C Explain how it is that two
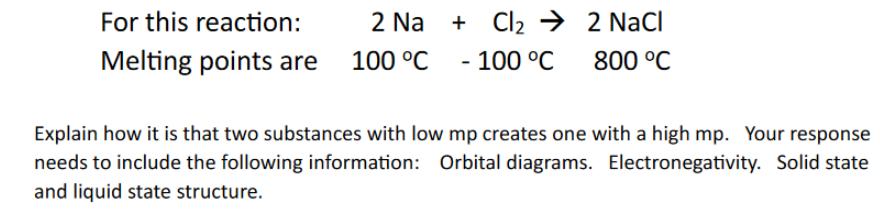
For this reaction: Melting points are 2 Na + Cl2 2 NaCl 100 C -100 C 800 C Explain how it is that two substances with low mp creates one with a high mp. Your response needs to include the following information: Orbital diagrams. Electronegativity. Solid state and liquid state structure.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The reaction between sodium Na and chlorine Cl2 to form sodium chloride NaCl is a classic example of an ionic bonding reaction In this reaction sodium ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


