Question: formula N 4) Argon is the third most abundant element in the atmosphere, while other noble gases trail behind (see table). Interestingly, both He and
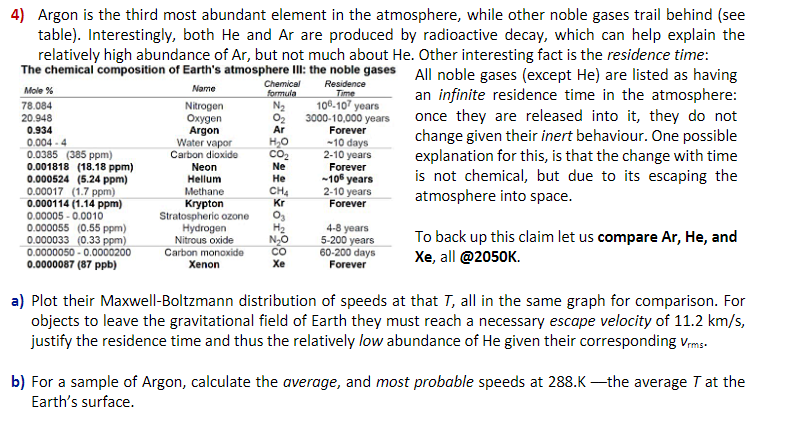
formula N 4) Argon is the third most abundant element in the atmosphere, while other noble gases trail behind (see table). Interestingly, both He and Ar are produced by radioactive decay, which can help explain the relatively high abundance of Ar, but not much about He. Other interesting fact is the residence time: The chemical composition of Earth's atmosphere III: the noble gases All noble gases (except He) are listed as having Chemical Name Mole % Residence Time an infinite residence time in the atmosphere: 78.084 Nitrogen 106-107 years 20.948 Oxygen 3000-10,000 years once they are released into it, they do not 0.934 Argon Ar Forever change given their inert behaviour. One possible 0.004. Water vapor HO -10 days 0.0385 (385 ppm) Carbon dioxide CO2 2-10 years explanation for this, is that the change with time 0.001818 (18.18 ppm) Neon Ne Forever 0.000524 (5.24 ppm) Hellum He -106 years is not chemical, but due to its escaping the 0.00017 (1.7 ppm) Methane CH4 2-10 years atmosphere into space. 0.000114 (1.14 ppm) Krypton Kr Forever 0.00005 -0.0010 Stratospheric ozone Og 0.000055 (0.55 ppm) Hydrogen 4-8 years 0.000033 (0.33 ppm) Nitrous oxide 5-200 years To back up this claim let us compare Ar, He, and 0.0000050 - 0.0000200 Carbon monoxide CO 60-200 days Xe, all @2050K. 0.0000087 (87 ppb) Xenon Forever NO a) Plot their Maxwell-Boltzmann distribution of speeds at that T, all in the same graph for comparison. For objects to leave the gravitational field of Earth they must reach a necessary escape velocity of 11.2 km/s, justify the residence time and thus the relatively low abundance of He given their corresponding Vrms. b) For a sample of Argon, calculate the average, and most probable speeds at 288.Kthe average T at the Earth's surface. formula N 4) Argon is the third most abundant element in the atmosphere, while other noble gases trail behind (see table). Interestingly, both He and Ar are produced by radioactive decay, which can help explain the relatively high abundance of Ar, but not much about He. Other interesting fact is the residence time: The chemical composition of Earth's atmosphere III: the noble gases All noble gases (except He) are listed as having Chemical Name Mole % Residence Time an infinite residence time in the atmosphere: 78.084 Nitrogen 106-107 years 20.948 Oxygen 3000-10,000 years once they are released into it, they do not 0.934 Argon Ar Forever change given their inert behaviour. One possible 0.004. Water vapor HO -10 days 0.0385 (385 ppm) Carbon dioxide CO2 2-10 years explanation for this, is that the change with time 0.001818 (18.18 ppm) Neon Ne Forever 0.000524 (5.24 ppm) Hellum He -106 years is not chemical, but due to its escaping the 0.00017 (1.7 ppm) Methane CH4 2-10 years atmosphere into space. 0.000114 (1.14 ppm) Krypton Kr Forever 0.00005 -0.0010 Stratospheric ozone Og 0.000055 (0.55 ppm) Hydrogen 4-8 years 0.000033 (0.33 ppm) Nitrous oxide 5-200 years To back up this claim let us compare Ar, He, and 0.0000050 - 0.0000200 Carbon monoxide CO 60-200 days Xe, all @2050K. 0.0000087 (87 ppb) Xenon Forever NO a) Plot their Maxwell-Boltzmann distribution of speeds at that T, all in the same graph for comparison. For objects to leave the gravitational field of Earth they must reach a necessary escape velocity of 11.2 km/s, justify the residence time and thus the relatively low abundance of He given their corresponding Vrms. b) For a sample of Argon, calculate the average, and most probable speeds at 288.Kthe average T at the Earth's surface
Step by Step Solution
There are 3 Steps involved in it
To address the given tasks well break them down into two parts a plotting the MaxwellBoltzmann distr... View full answer

Get step-by-step solutions from verified subject matter experts


