Question: Four proteins are dissolved in solution. The constants in the Cohn equation for the proteins are given in the table below along with the concentration
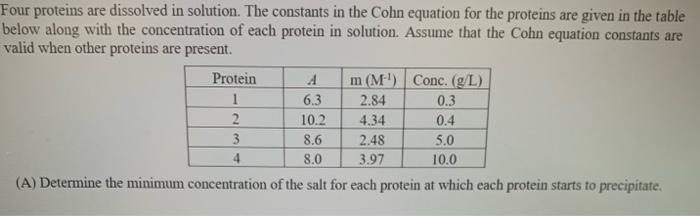

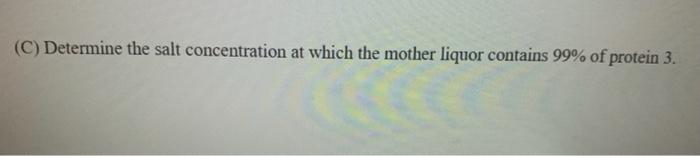
Four proteins are dissolved in solution. The constants in the Cohn equation for the proteins are given in the table below along with the concentration of each protein in solution. Assume that the Cohn equation constants are valid when other proteins are present. Protein A m (M) Conc. (g/L) 1 6.3 2.84 0.3 2 10.2 4.34 0.4 3 8.6 2.48 5.0 4 8.0 3.97 10.0 (A) Determine the minimum concentration of the salt for each protein at which each protein starts to precipitate. (B) Determine the maximum salt concentration at which the maximum percentage of recovery of protein 4 with the highest purity in precipitate can be obtained. (C) Determine the salt concentration at which the mother liquor contains 99% of protein 3. C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


