Question: Four vapor pressure data points-two representing solid-vapor equilibrium and two representing liquid-vapor equilibrium-are available for a compound: The molar volume of the compound is VS=
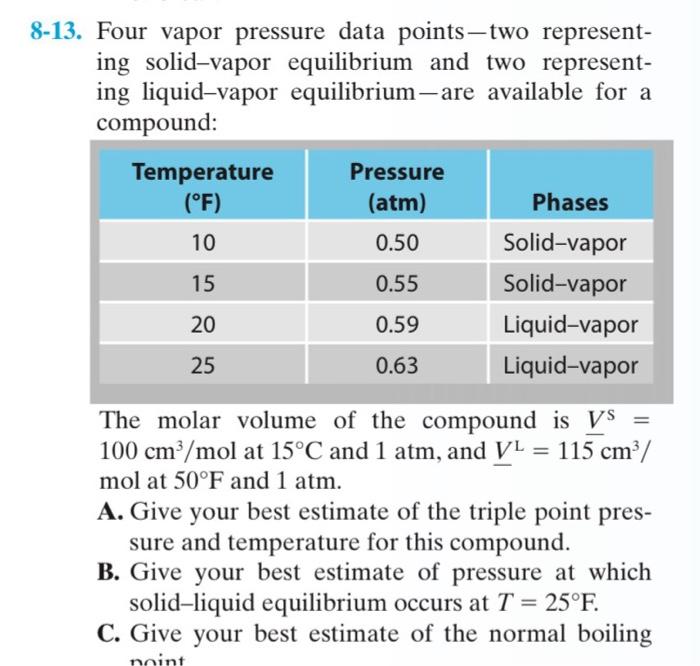
Four vapor pressure data points-two representing solid-vapor equilibrium and two representing liquid-vapor equilibrium-are available for a compound: The molar volume of the compound is VS= 100cm3/mol at 15C and 1atm, and VL=115cm3/ mol at 50F and 1atm. A. Give your best estimate of the triple point pressure and temperature for this compound. B. Give your best estimate of pressure at which solid-liquid equilibrium occurs at T=25F. C. Give your best estimate of the normal boiling
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


