Four vapor pressure data pointstwo representing solidvapor equilibrium and two representing liquidvapor equilibriumare available for a compound:
Question:
Four vapor pressure data points—two representing solid–vapor equilibrium and two representing liquid–vapor equilibrium—are available for a compound:
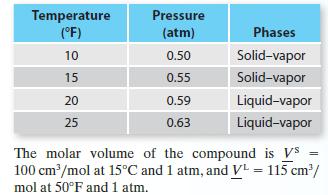
A. Give your best estimate of the triple point pressure and temperature for this compound.
B. Give your best estimate of pressure at which solid–liquid equilibrium occurs at T = 25°F.
C. Give your best estimate of the normal boiling point.
Transcribed Image Text:
Temperature (°F) 10 15 20 25 Pressure (atm) 0.50 0.55 0.59 0.63 Phases Solid-vapor Solid-vapor Liquid-vapor Liquid-vapor The molar volume of the compound is Vs = 100 cm³/mol at 15°C and 1 atm, and V¹ = 115 cm³/ mol at 50°F and 1 atm.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Four vapor pressure data pointstwo representing solidvapor equilibrium and two representing liquidvapor equilibriumare available for a compound: A. Give your best estimate of the triple point...
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
A compound has a molecular mass of 120 g/mol, and the information in the table below is the only other data available for a compound. Fill in all of the empty cells with your best estimate of the...
-
Methanol, CH3OH, is prepared industrially from the gasphase catalytic balanced reaction that has been depicted here using molecular models. In a laboratory test, a reaction vessel was filled with...
-
1. Compare GEs approach to performance evaluation with that of ICI (mentioned in the chapter). 2. Critically evaluate the strengths and weaknesses of each companys approach to the performance...
-
Would you expect the following variables to be procyclical or countercyclical if GDP was above trend? Explain. a. inflation b. unemployment c. employment d. real wages e. nominal interest rates
-
Using Program15.m (central difference method), solve Problem 11.20. Data From Problem 11.20:- The equations of motion of a two-degree-of-freedom system are given by \(2 \ddot{x}_{1}+6 x_{1}-2...
-
A chemical reaction A ? B is carried out in a closed vessel. The following data are taken for the concentration of A, CA (g/L), are a function of time, t (min, form the start of the reaction: A...
-
Windsor Company's income statement for the year ended December 31, 2025, contained the following condensed information. Service revenue $843,000 Operating expenses (excluding depreciation) $622,000...
-
This problem involves the same compound that was examined in Problems 6-14 through 6-17, which in the vapor phase was described by the EOS: A. The fugacity in the vapor phase at T = 50C and P = 0.1...
-
Estimate the vapor pressure of ethanol at temperatures of T = 0, 50, 100, and 150C, using the following methods. A. The Antoine equation B. The Clausius-Clapeyron equation with H vap = 42.0 kJ/mol...
-
In an experiment to compare two different surgical procedures for hernia repair (A Single-Blinded, Randomized Comparison of Laparoscopic Versus Open Hernia Repair in Children, Pediatrics [2009]: 332...
-
Hysteretic damping can be modeled using a differential equation with a complex stiffness. Indicate whether the statement presented is true or false. If true, state why. If false, rewrite the...
-
An operation consists of two steps, the first of which has a reliability of 97% and the second a reliability of 99%. What is the probability that the operation will fail?
-
A seismometer actually measures the displacement of the seismic mass relative to the displacement of the body the instrument is set up to measure. Indicate whether the statement presented is true or...
-
The equation for the response of a system with hysteretic damping is nonlinear in general but is linear when the system is subject to a single-frequency excitation. Indicate whether the statement...
-
An AC source with \(\mathscr{E}_{m}=220 \mathrm{~V}\) AC has a frequency of \(50.0 \mathrm{~Hz}\) and produces the same maximum current in two series circuits. Each circuit contains a \(160-\Omega\)...
-
What is meant by the term functional currency?
-
You are standing at x = 9.0 km and your assistant is standing at x = 3.0 km. Lightning bolt 1 strikes at x = 0 km and lightning bolt 2 strikes at x = 12.0 km. You see the flash from bolt 2 at t = 10...
-
The following represents pressure samples, in pounds per square inch (psi), taken in a fuel line once every second for 10 sec. a. Fit a first-degree polynomial, a second-degree polynomial, and a...
-
A liquid boils when its vapor pressure equals the external pressure acting on the surface of the liquid. This is why water boils at a lower temperature at higher altitudes. This information is...
-
The solubility of salt in water is a function of the water temperature. Let S represent the solubility of NaCl (sodium chloride) as grams of salt in 100 g of water. Let T be temperature in C. Use the...
-
The accounting records of Cullumber Electronics show the following data. Beginning inventory 3 , 0 0 0 units at $ 4 Purchases 9 , 5 0 0 units at $ 6 Sales 1 0 , 1 0 0 units at $ 9 Determine cost of...
-
The demand for one of the major products for a company sustained large and continuing declines. As a result, the company performed an asset impairment test on the fixed assets that were associated...
-
what are the efficacious strategies for orchestrating structural transformations within complex organizational ecosystems, ensuring congruence between strategic imperatives, organizational...

Study smarter with the SolutionInn App


