Question: FOURTH TIME POSTING ANSWER IS NOT 0.029 L OR 0.030 L OR 0.52 L The small bubbles that form on the bottom of a Use
FOURTH TIME POSTING ANSWER IS NOT 0.029 L OR 0.030 L OR 0.52 L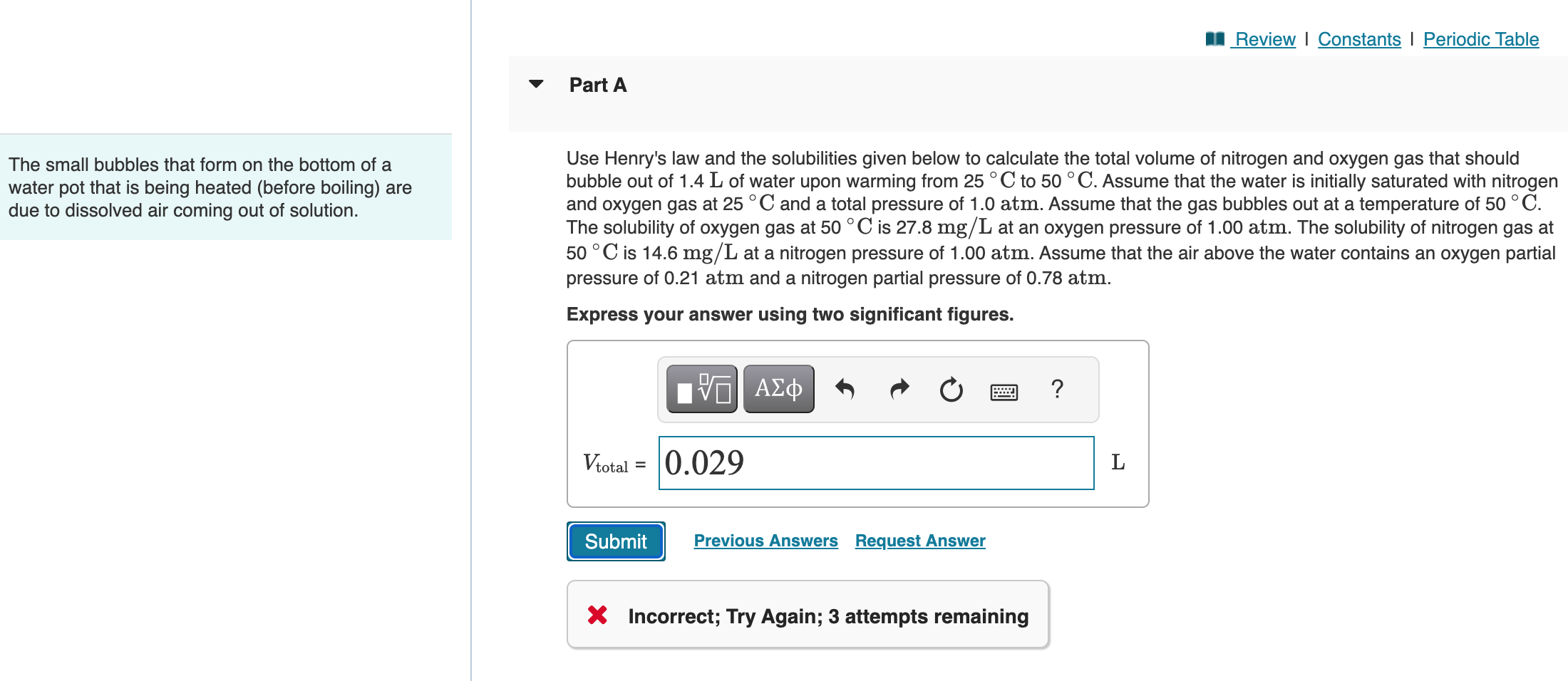
The small bubbles that form on the bottom of a Use Henry's law and the solubilities given below to calculate the total volume of nitrogen and oxygen gas that should water pot that is being heated (before boiling) are bubble out of 1.4L of water upon warming from 25C to 50C. Assume that the water is initially saturated with nitrogen due to dissolved air coming out of solution. and oxygen gas at 25C and a total pressure of 1.0atm. Assume that the gas bubbles out at a temperature of 50C. The solubility of oxygen gas at 50C is 27.8mg/L at an oxygen pressure of 1.00atm. The solubility of nitrogen gas at 50C is 14.6mg/L at a nitrogen pressure of 1.00atm. Assume that the air above the water contains an oxygen partial pressure of 0.21atm and a nitrogen partial pressure of 0.78atm. Express your answer using two significant figures. X Incorrect; Try Again; 3 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


