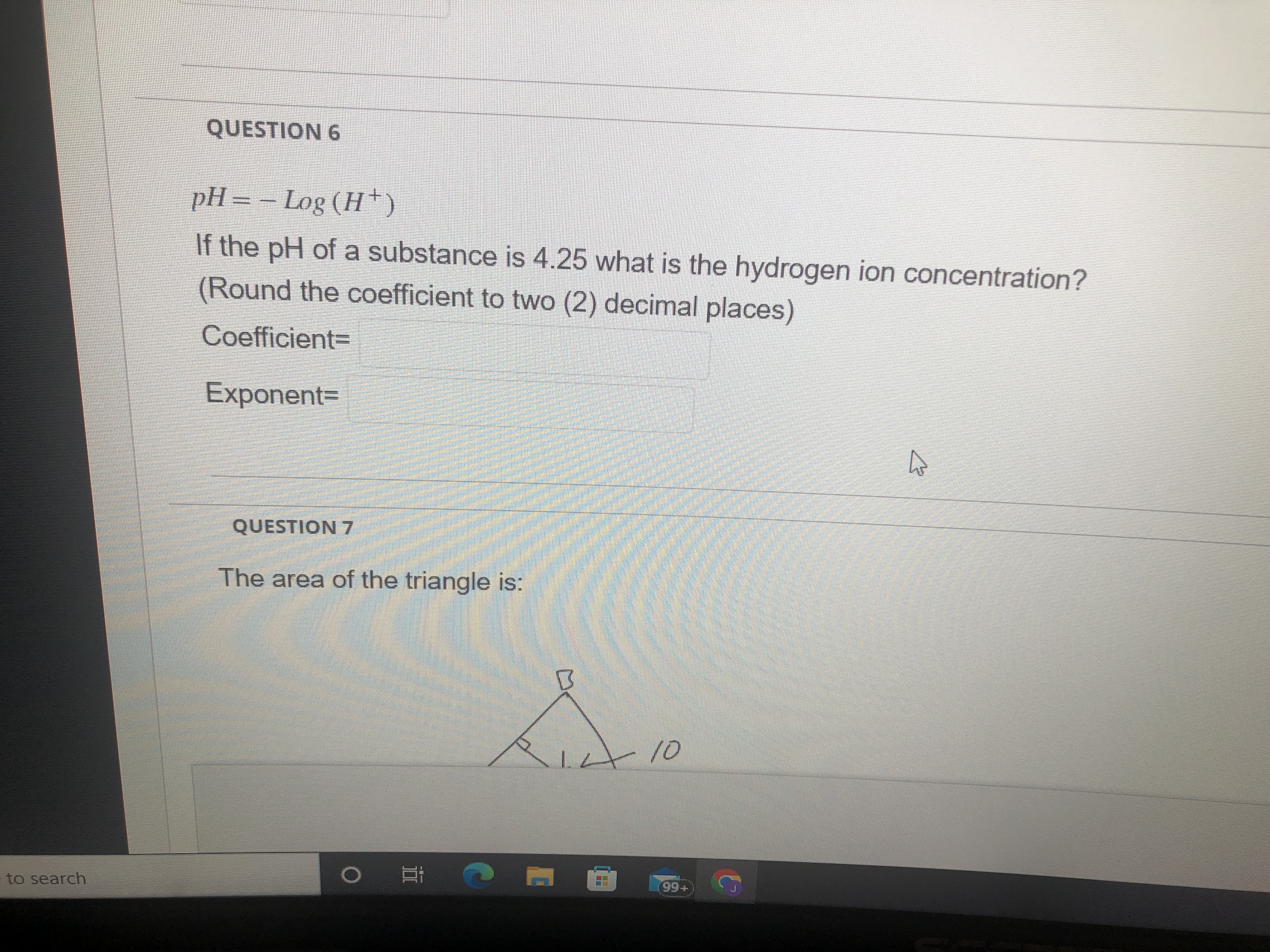
Question: fQUESTION 6 PH = - Log ( H +) If the pH of a substance is 4.25 what is the hydrogen ion concentration? (Round the
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


