Question: Freezing point depression (C),T, molality (m) of solution containing unknown Number of moles (moles) of unknown used Molecular weight (g/mole) of unknown Freezing point depression
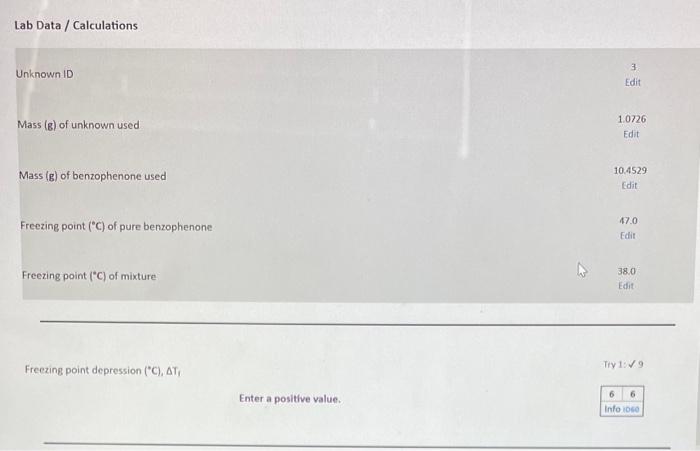

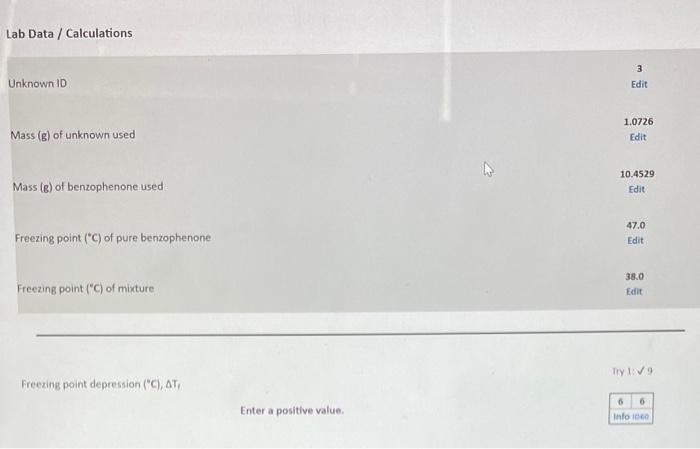

Freezing point depression (C),T, molality (m) of solution containing unknown Number of moles (moles) of unknown used Molecular weight (g/mole) of unknown Freezing point depression ("C), Tr molality (m) of solution containing unknown Number of moles (moles) of unknown used Molecular weight (g/mole) of unknown
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


