Question: Freezing Point Depression Report Sheet Name and Drawer Number Robert Allbritton #46 Partner's Name Carlos Rotinsen Sample number Freezing point of pure acetic acid after
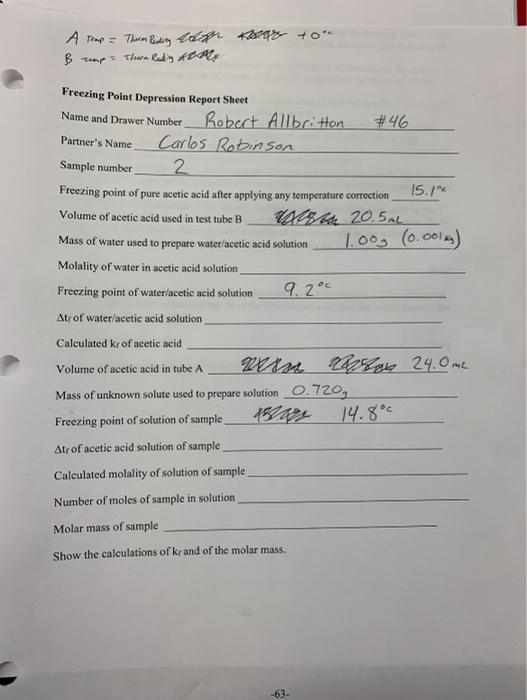

Freezing Point Depression Report Sheet Name and Drawer Number Robert Allbritton \#46 Partner's Name Carlos Rotinsen Sample number Freezing point of pure acetic acid after applying any temperature correction 15. 1nc Volume of acetic acid used in test tube B Mass of water used to prepare water/acetic acid solution 1.003(0.001sg) Molality of water in acetic acid solution Freezing point of water/acetic acid solution 9.2 Atr of water/acetic acid solution Calculated kr of acetic acid Volume of acetic acid in tube A 24.0m2 Mass of unknown solute used to prepare solution 0.720g Freezing point of solution of sample 14.8 Atr of acetic acid solution of sample Calculated molality of solution of sample Number of moles of sample in solution Molar mass of sample Show the calculations of kr and of the molar mass. 3. Use the freezing points determined in step 2 of the Caleulations and the freezing point of pure acetic acid to calculate the freezing point depressions t t for the two solutions. Use Equation (82) for the calculations. 4. Use the volume of water ( 1.00mL ) added to test tube B (step 10 of Procedure), the density of water (1.00g/mL) and the molar mass of water (18.0g/mol) to calculate the moles of added 5. Use the volume of acetic acid in test tube B (Procedure, step 4) and the density of acetic acid (1.049g/mL) to ealculate the mass (kg) of acetic acid in tube B. 6. Calculate the molality of water in the acetic acid in tube B by dividing the moles of water calculated in step 4 of the Caleulations by the mass (kg) of acetic acid ealculated in step 5. 7. Use the water molality and the freezing point depression for the water/acetic acid solution to calculate the freezing point depression constant kr by using Equation (81). 8. Use the freezing point depression for the sample solution and the freezing point depression constant calculated in step 7 of the Calculations to calculate the molality of the prepared solution of the sample. 9. Use the molality of the solution of the sample and the mass of solvent (acetic acid) in the sample solution to calculate the number of moles of the sample in the solution. Equation (8-3) can be used for the calculation where m is the molality of the compound in the solution, n is the number of moles of the compound and kg is the mass of the solvent in kilograms. molality, m=n/kg (83) 10. Using the number of moles of the sample in the solution and the mass w of the compound that was used to prepare the solution (step 14 of the Procedure), calculate the molar mass of the compound by using Equation (8-4). molarmass=mass(g) 11. Transfer all requested information to the Report Sheet
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


