Question: - Ga3 and Ge44 are two important cations for the semiconductor industry. Using the table to the right and the relative size rules, determine if
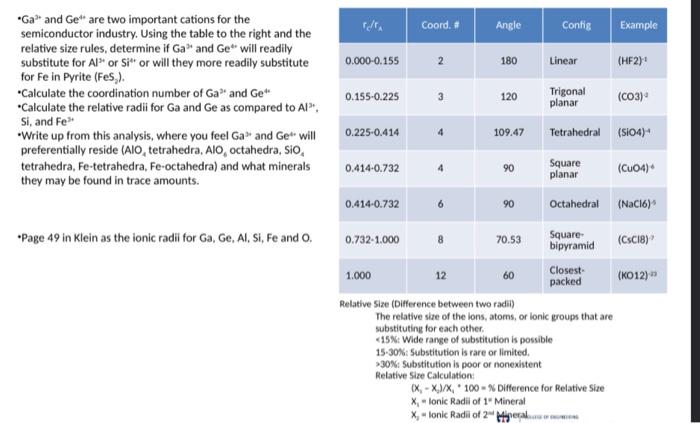
- Ga3 and Ge44 are two important cations for the semiconductor industry. Using the table to the right and the relative size rules, determine if Ga37 and Ge*t will readily substitute for Al3 or Si4 or will they more readily substitute for Fe in Pyrite (FeS 2 ). -Calculate the coordination number of Ga3 and Ge4 -Calculate the relative radii for Ga and Ge as compared to Al3* : Si, and Fe3 "Write up from this analysis, where you feel Ga2 and Ge* will preferentially reside ( AlO4 tetrahedra, AlO4 octahedra, SiO4 tetrahedra, Fe-tetrahedra, Fe-octahedra) and what minerals they may be found in trace amounts. -Page 49 in Klein as the ionic radii for Ga, Ge, Al, Si, Fe and O. Relative Size (Difference between two radii) The relative she of the ions, atoms, or lonic groups that are substituting for each other. 15N: Wide range of substitution is possible 15-30\%: Substitution is rare or limited. >30N: Substitution is poor or nonexistent Relative Size Calculation: (x1x3)/x4+100=WifferenceforRelativeSizex1=lonicRadiliof1=Mineralxy=lonicRadiliof2-Mhperal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


