Question: Gas (A) is dissolved in a liquid solvent (S) with a composition of 21% by mole. The solvent is stripped to remove the gas from
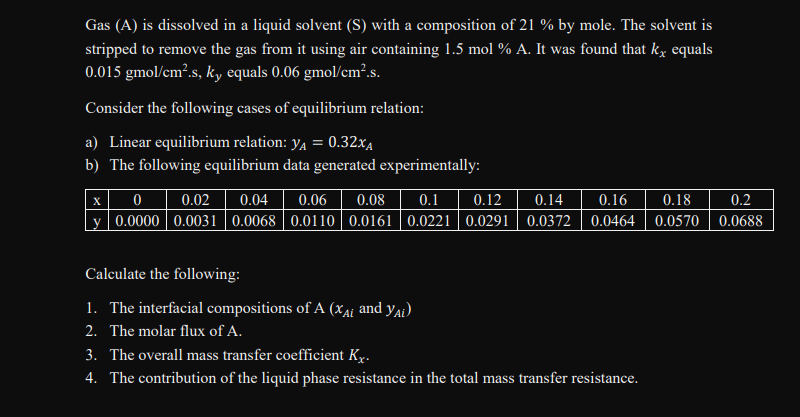
Gas (A) is dissolved in a liquid solvent (S) with a composition of 21% by mole. The solvent is stripped to remove the gas from it using air containing 1.5mol%A. It was found that kx equals 0.015gmol/cm2.s,ky equals 0.06gmol/cm2.s. Consider the following cases of equilibrium relation: a) Linear equilibrium relation: yA=0.32xA b) The following equilibrium data generated experimentally: Calculate the following: 1. The interfacial compositions of A(xAi and yAi) 2. The molar flux of A. 3. The overall mass transfer coefficient Kx. 4. The contribution of the liquid phase resistance in the total mass transfer resistance
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


