Question: Gaseous indium dihydride is formed from the elements at elevated temperature: Calculate Qp In(g)+H2(g)InH2(g),Kp=1.48 at 973K Express the reaction quotient to three significant figures. The
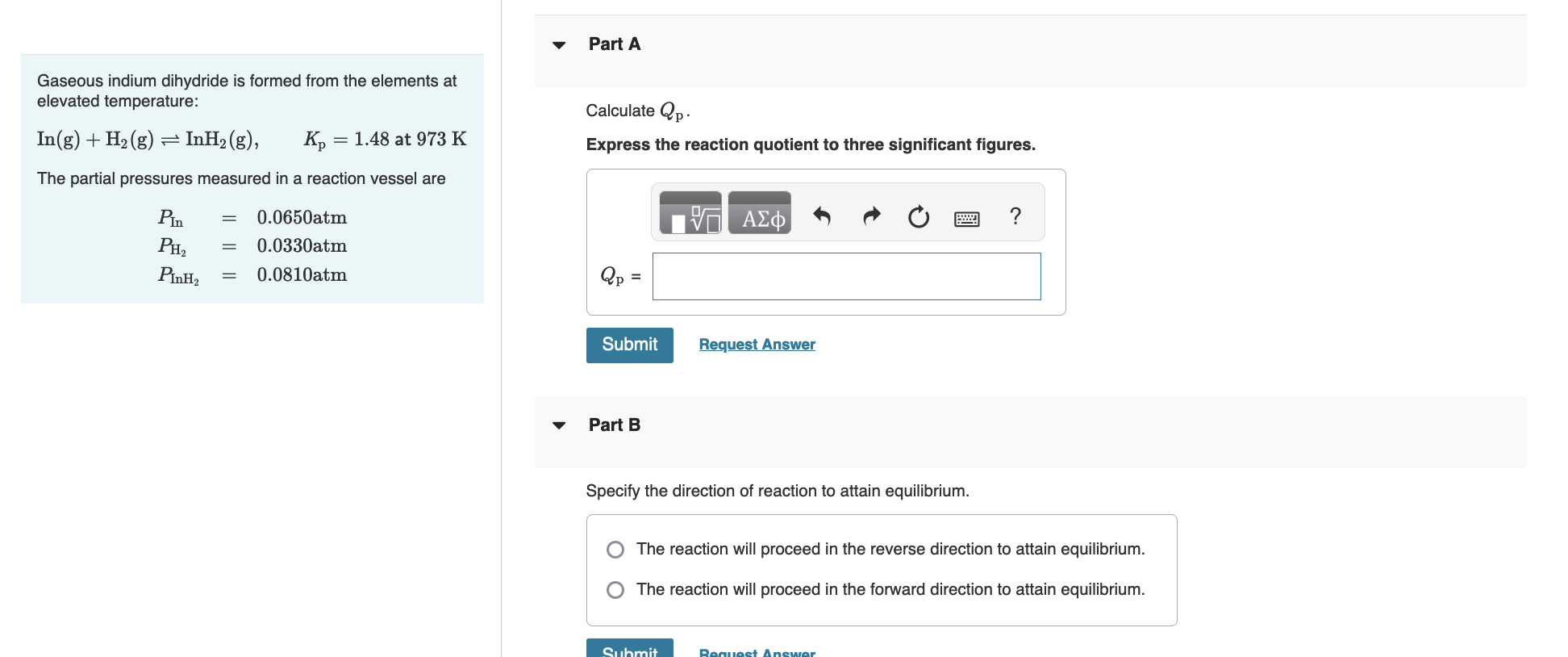
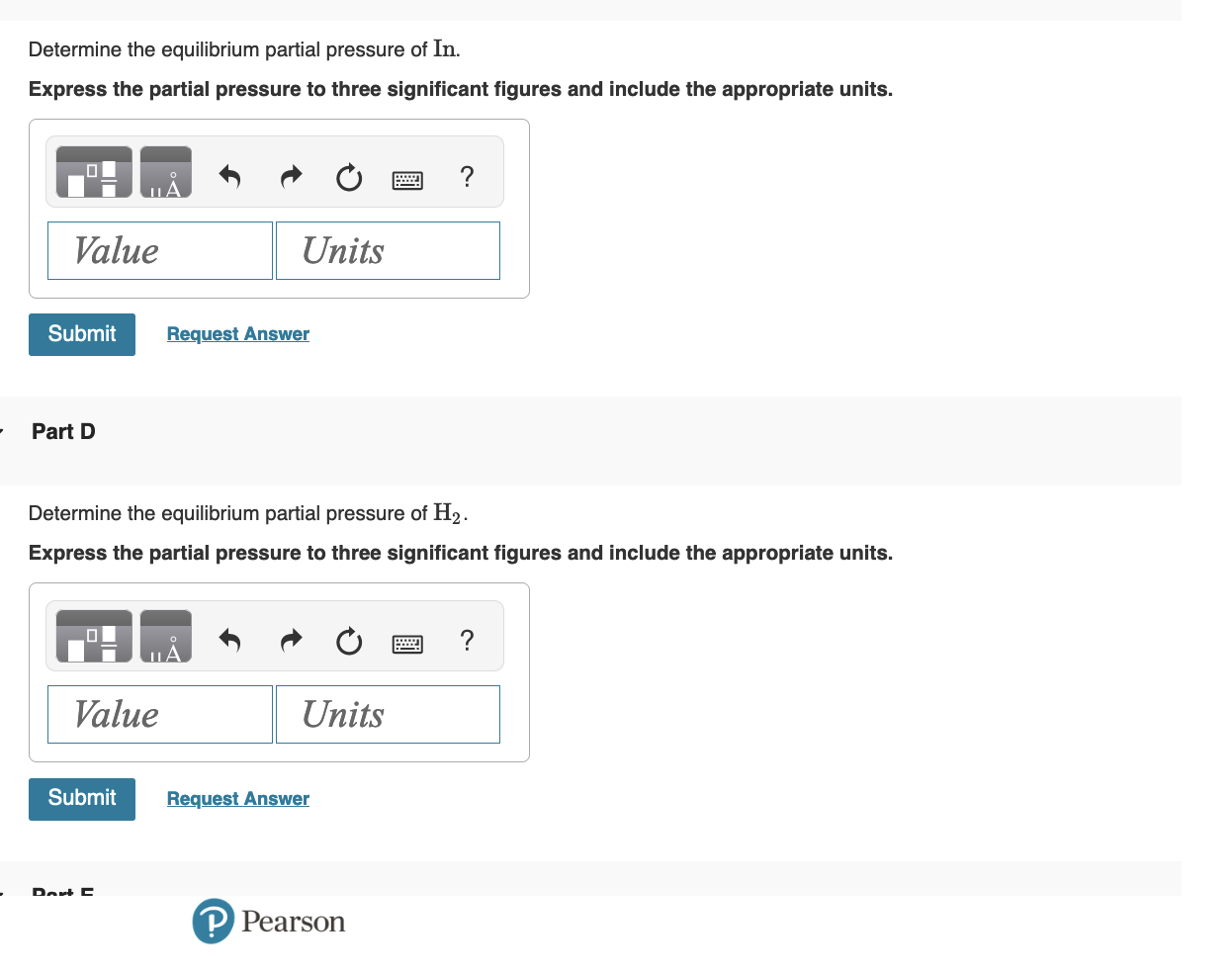
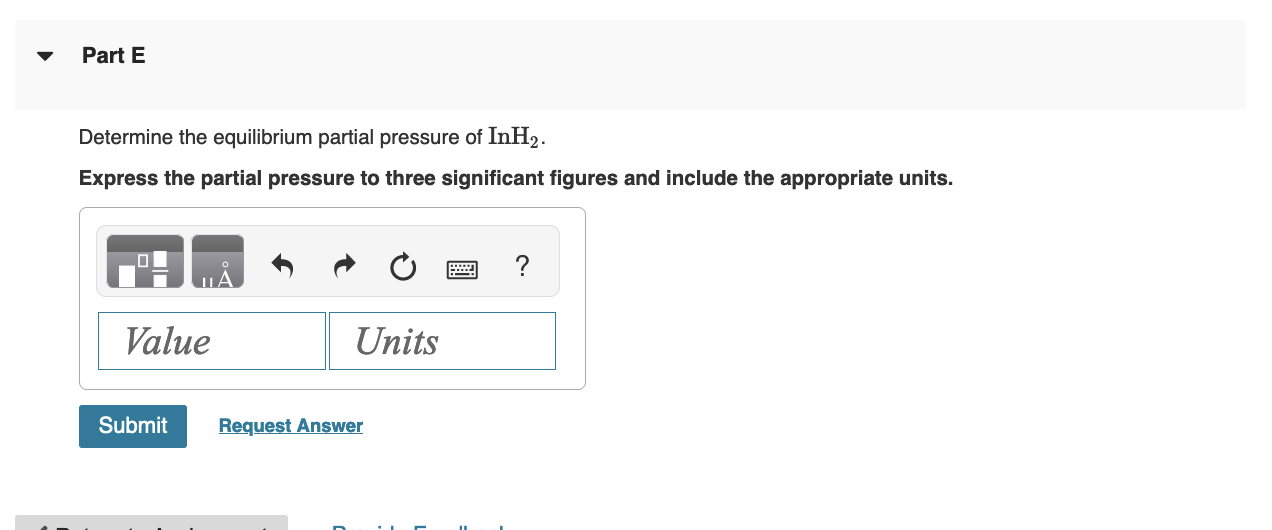
Gaseous indium dihydride is formed from the elements at elevated temperature: Calculate Qp In(g)+H2(g)InH2(g),Kp=1.48 at 973K Express the reaction quotient to three significant figures. The partial pressures measured in a reaction vessel are PIn=0.0650atmPH2=0.0330atmPInH2=0.0810atmQp= Part B Specify the direction of reaction to attain equilibrium. The reaction will proceed in the reverse direction to attain equilibrium. The reaction will proceed in the forward direction to attain equilibrium. Determine the equilibrium partial pressure of In. Express the partial pressure to three significant figures and include the appropriate units. Part D Determine the equilibrium partial pressure of H2. Express the partial pressure to three significant figures and include the appropriate units. Determine the equilibrium partial pressure of InH2. Express the partial pressure to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


