Question: Gaseous reactant A diffuses through a gas film and reacts on the surface of a solid according to a reversible first order rate, -TA=k (CAS-C
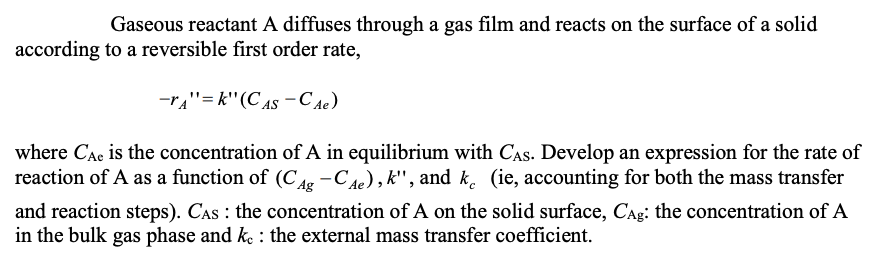
Gaseous reactant A diffuses through a gas film and reacts on the surface of a solid according to a reversible first order rate, -TA"=k" (CAS-C Ae) where CAC is the concentration of A in equilibrium with CAS. Develop an expression for the rate of reaction of A as a function of (CAg-Ce), k", and kc (ie, accounting for both the mass transfer and reaction steps). CAs the concentration of A on the solid surface, Cag: the concentration of A in the bulk gas phase and ke: the external mass transfer coefficient
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


