Gaseous reactant A diffuses through a gas film and reacts on the surface of a solid according
Question:
Gaseous reactant A diffuses through a gas film and reacts on the surface of a solid according to a reversible first-order rate,
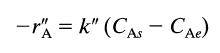
where CA, is the concentration of A in equilibrium with the solid surface. Develop an expression for the rate of reaction of A accounting for both the mass transfer and reaction steps.
Transcribed Image Text:
-ra= k" (CAS- CAe) A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
In order to develop an expression that accounts for both the mass transfer and reaction steps we need to consider the two different rates 1 The mass t...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
In chemical vapor deposition (CVD), a semiconducting or insulating solid material is formed in a reaction between a gaseous species and a species adsorbed on the surface of silicon wafers (disks...
-
The Corporate Average Fuel Economy (CAFE) regulations were put into law by Congress in 1975 to promote the sale of fuel-efficient automobiles and light trucks. The law requires automakers to boost...
-
Discuss the advantages and disadvantages of one of the financial innovations you select. (For example, sweep accounts, junk bond, smart card etcSelect one of them, then explain briefly and afterwards...
-
An article in Sociological Methods & Research (May 2001) analyzed the data presented in the accompanying table. A sample of 262 Kansas pig farmers was classified according to their education level...
-
What problems do analysts often encounter when they try to implement an ERP package?
-
A gas expands and does P - V work on the surroundings equal to 325 J. At the same time, it absorbs 127 J of heat from the surroundings. Calculate the change in energy of the gas?
-
Compare the objectives of a product to its features.
-
A metropolitan childrens museum open year-round wants to see if the variance in daily attendance differs between the summer and winter months. Random samples of 30 days each were selected and showed...
-
9. If f(x)=In x, then lim f(x)-(3) is 813 413
-
In slurry reactors, pure reactant gas is bubbled through liquid containing suspended catalyst particles. Let us view these kinetics in terms of the film theory, as shown in Fig. P17.3. Thus, to reach...
-
Aqueous A (C AO = 50 mol/m 3 ) with physical properties close to water (p =1000 kg/m 3 , D -9 = m 2 /s) reacts by a second-order reaction (k = m 3 /mol s) as it flows at 10 mm/s through a tubular...
-
Solve each equation by factoring. x 2 + 5x - 6 = 0
-
show calculations 3 Inputs 4 discount rate 5 annual net revenue growth rate 6 Initial investment 7 revenue (year 1) 8 9 10 11 12 year 0 13 year 1 14 year 2 15 year 3 16 year 4 17 year 5 18 year 6 19...
-
Give two examples of even functions and two examples of odd functions that are NOT polynomial. Give the equations and graphs (you can sketch them on the same set of axis below. -10 -$ flay-10 -51 -10...
-
Let ECR be an open set and let f:ER be Continuously differentiable. Suppose further there exists (x, y) EE such that af (xo, yo) #0 ax Prove that is not injective. Hint: If G" (u, v) = (4(u, v), y(u,...
-
Consult the spreadsheet entitled "Walmart Cost of Capital Supplement." Give an explanation of how Dale and Lee have calculated Walmart's cost of capital. Do you agree with their estimate of Walmart's...
-
Currently, Teewinot sells 1,500,000 per year, but they expect demand to increase by 10% each year for the next 4 years then drop down to 3% growth in volume for the remaining life of the machine....
-
Below are pairs of changes that affect elements of the accounting equation. Give an example of a transaction or economic event that reflects each: a. Asset increases, asset decreases b. Asset...
-
Anne is employed by Bradley Contracting Company. Bradley has a $1.3 million contract to build a small group of outbuildings in a national park. Anne alleges that Bradley Contracting has discriminated...
-
Go to the five LearnChemE screencasts link for Chapter 5 (http://www.umich.edu/~elements/6e/05chap/learn-cheme-videos.html). 1. In the screencast of the PBR with pressure drop, is there a problem...
-
How would you modify Table 6-2 for a. A constant-volume gas-phase reaction, and b. A variable-volume gas-phase reaction? Table 6-2 1. Mole balances: BR dNAdt=rAV dNBdt=rBV dNCdt=rCV dNDdt=rDV PFR PBR...
-
(a) Without referring back, make a list of the most important items you learned in this chapter. (b) Overall, what do you believe were the three major purposes of the chapter?
-
Cost Reduction Examine a scenario where a company needs to reduce production costs without compromising product quality. How can TQM methodologies be employed to achieve cost savings while...
-
1.What is health care management ?2.What meant by health care management ?3.Why is health care management important ?4.What is scope of health care management ?5.What is meaning of health care ?
-
Define consideration. Under what circumstances is the object or consideration of a contract deemed unlawful? Give illustrations.

Study smarter with the SolutionInn App


