Question: General Guidelines for Design Exercises (Problem 3-4) - Design a steady-state continuous process. - Assume liquid-gas separations are perfect if boiling points differ by more


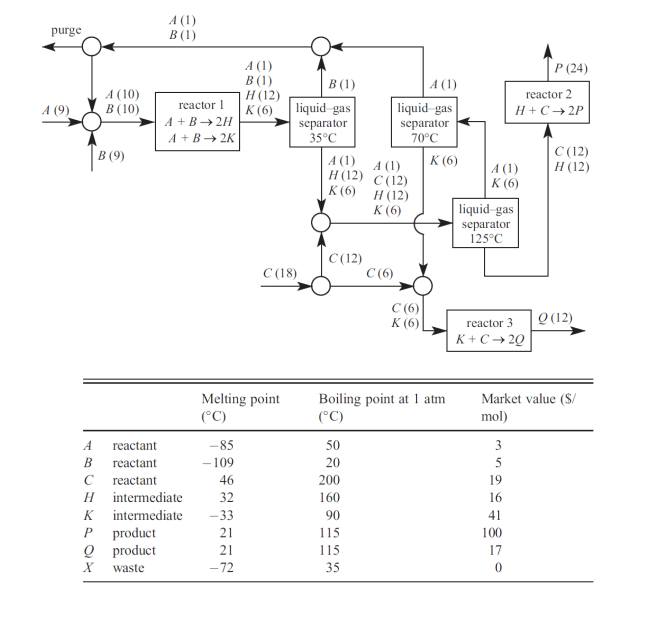
General Guidelines for Design Exercises (Problem 3-4) - Design a steady-state continuous process. - Assume liquid-gas separations are perfect if boiling points differ by more than 3C. - Solid-liquid separations produce wet solids. - Assume reactions occur only in reactors. - Assume no reactions other than the reactions given. - Unless instructed otherwise, you may neglect heat exchangers, heaters, coolers, and pumps. - In commercial chemical processes, a catalyst is typically a porous and/or granular solid. The catalyst is a permanent component of the reactor; there is no catalyst in the product stream. The catalyst is not consumed in the reaction; there is no catalyst in the feed stream. Unless stated otherwise, assume no catalyst is present in a process stream. - Many designs are workable. Explore different options and choose your best design. General Flowsheet Practice - Use a discrete unit for each operation. For example, a reactor should not do double duty as a mixer and/or a separator. A reactor should have exactly one input and one output. - Label each unit. For reactors, indicate the reaction, such as "hydrolysis reactor." For separators, indicate the physical basis of the separation, such as "liquid-gas separator." Where appropriate, indicate the temperature in the unit. You need not label combiners and splitters. You need not label mixers and heat exchangers if you use descriptive symbols (a stirring paddle or a zigzag stream, respectively). - List the substances in every stream and state approximate flow rates. One significant figure is sufficient. - Where appropriate, list the physical state of the substances - gas, solid, liquid, or aq (in aqueous solution). - Indicate the destination of any stream that leaves the process (for example, "vent to air," "to landfill," or "to market"). - Use conventions to improve the readability of your flowsheet. For a liquid-gas separator the gas should leave the top and the liquid should leave the bottom. If convenient, the process should start in the upper left-hand corner. It is helpful if streams entering the process start at the periphery and streams leaving the process terminate at the periphery. 3. The process below produces two chemical products, P and Q, by the following reactions. First, we form the intermediate H : A+B2Hreactionis60%complete. We then react intermediate H with C to form a product P : H+C2Preactionis100%complete: We must avoid the reaction of B and C to form X : B+C2Xreactionis90%complete. There is a complication. A and B have a parallel reaction that forms K : A+B2Kreactionis30%complete. But we can react K with C to form a product Q : K+C2Qreactionis100%complete. Because P and Q cannot be separated, we must form Q in a separate reactor. The process below is viable and the molar flow rates (given by the numbers in parentheses) are correct. However, the process can be improved. Suggest at least three improvements to reduce costs or increase revenue. You might, for example, eliminate a unit, or decrease the flow rate through a unit, or decrease a reactant flow rate. You need not calculate flow rates in your improved process. Design Rule - Use no more than 9mol/min of reactant A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


