Question: General Instructions: 1) Answer each word problem using the complete steps in problem-solving, if applicable. 2) Apply the rules of significant figures for all of
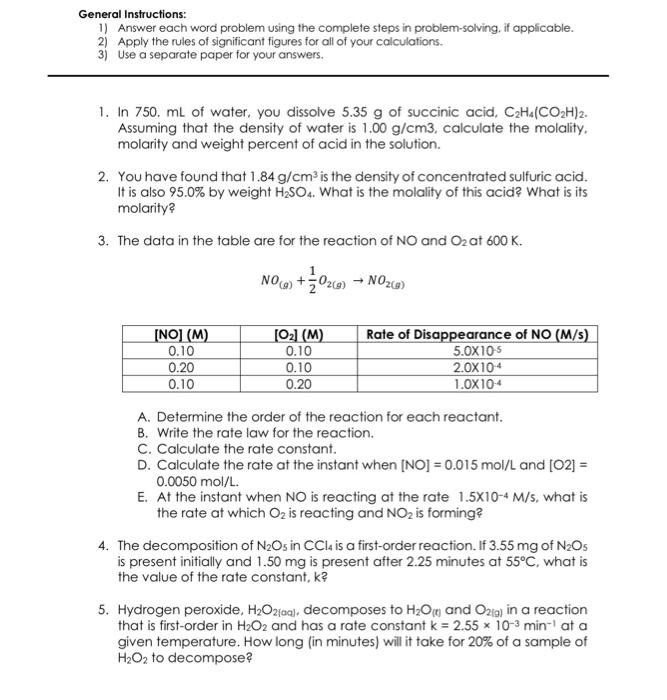
General Instructions: 1) Answer each word problem using the complete steps in problem-solving, if applicable. 2) Apply the rules of significant figures for all of your calculations. 3) Use a separate paper for your answers. 1. In 750. mL of water, you dissolve 5.35g of succinic acid, C2H4(CO2H)2. Assuming that the density of water is 1.00g/cm3, calculate the molality, molarity and weight percent of acid in the solution. 2. You have found that 1.84g/cm3 is the density of concentrated sulfuric acid. It is also 95.0% by weight H2SO4. What is the molality of this acid? What is its molarity? 3. The data in the table are for the reaction of NO and O2 at 600K. NO(g)+21O2(g)NO2(g) A. Determine the order of the reaction for each reactant. B. Write the rate law for the reaction. C. Calculate the rate constant. D. Calculate the rate at the instant when [NO]=0.015mol/L and [O2]= 0.0050mol/L. E. At the instant when NO is reacting at the rate 1.5104M/s, what is the rate at which O2 is reacting and NO2 is forming? 4. The decomposition of N2O5 in CCl4 is a first-order reaction. If 3.55mg of N2O5 is present initially and 1.50mg is present after 2.25 minutes at 55C, what is the value of the rate constant, k ? 5. Hydrogen peroxide, H2O2[(a), decomposes to H2O(t) and O2(j) in a reaction that is first-order in H2O2 and has a rate constant k=2.55103min1 at a given temperature. How long (in minutes) will it take for 20% of a sample of H2O2 to decompose
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


