Question: i need help for the 6 questions plis Table 1: Data and Observations Table 2: Mass of CaCl2 after 24 Hours 1. Calculate the theoretical
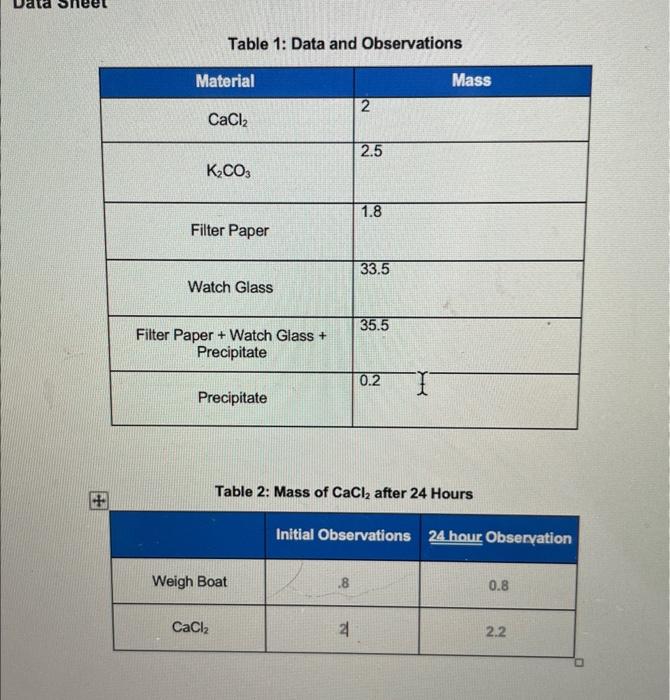

Table 1: Data and Observations Table 2: Mass of CaCl2 after 24 Hours 1. Calculate the theoretical yield of the solid precipitate. Show your work. 2. Use Question 1 to calculate the percent yield of the solid precipitate. Show your work. 3. Based on your yield of calcium carbonate, calculate the mass of calcium present in the original solution. 4. Analyze the data and use your yield of calcium carbonate to determine the experimental concentration of calcium chloride in the solution that you made in steps 6 and 7 . Show all calculations and report in %wt/v concentration. 5. Calculate the theoretical concentration of calcium chloride in the solution that you made up in steps 6 and 7 . Show all calculations and reportin % wot/ concentration. 6. Using your answers from questions 4 and 5 , create a bar graph comparing the theoretical to experimental percent concentrations of calcium chloride in the solution. Then, calculate percent error between the two concentrations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


