Question: Get me the matlab code foreach and every step..The reactions and kinetic rate equations for two parallel ( competing ) reactions are given as follows:
Get me the matlab code foreach and every step..The reactions and kinetic rate equations for two parallel competing
reactions are given as follows:
The initial concentration of is given as moles rates
and in per hour. The concentration equations for each species
with respect to time are given by;
Plot the concentration profiles of and with time time
increments hour pts
If is the desired product, at what time will its production be
maximized? pts
Evaluate the selectivity at this point. pts
Evaluate the yield of and at this point. pts
Also, solve this problem using probabilistic methods Simulated
Annealing or Genetic Algorithm and compare your solutions in
parts bc and d pts
Selectivity Desired productUndesired product
Yield Product formedReactant consumed
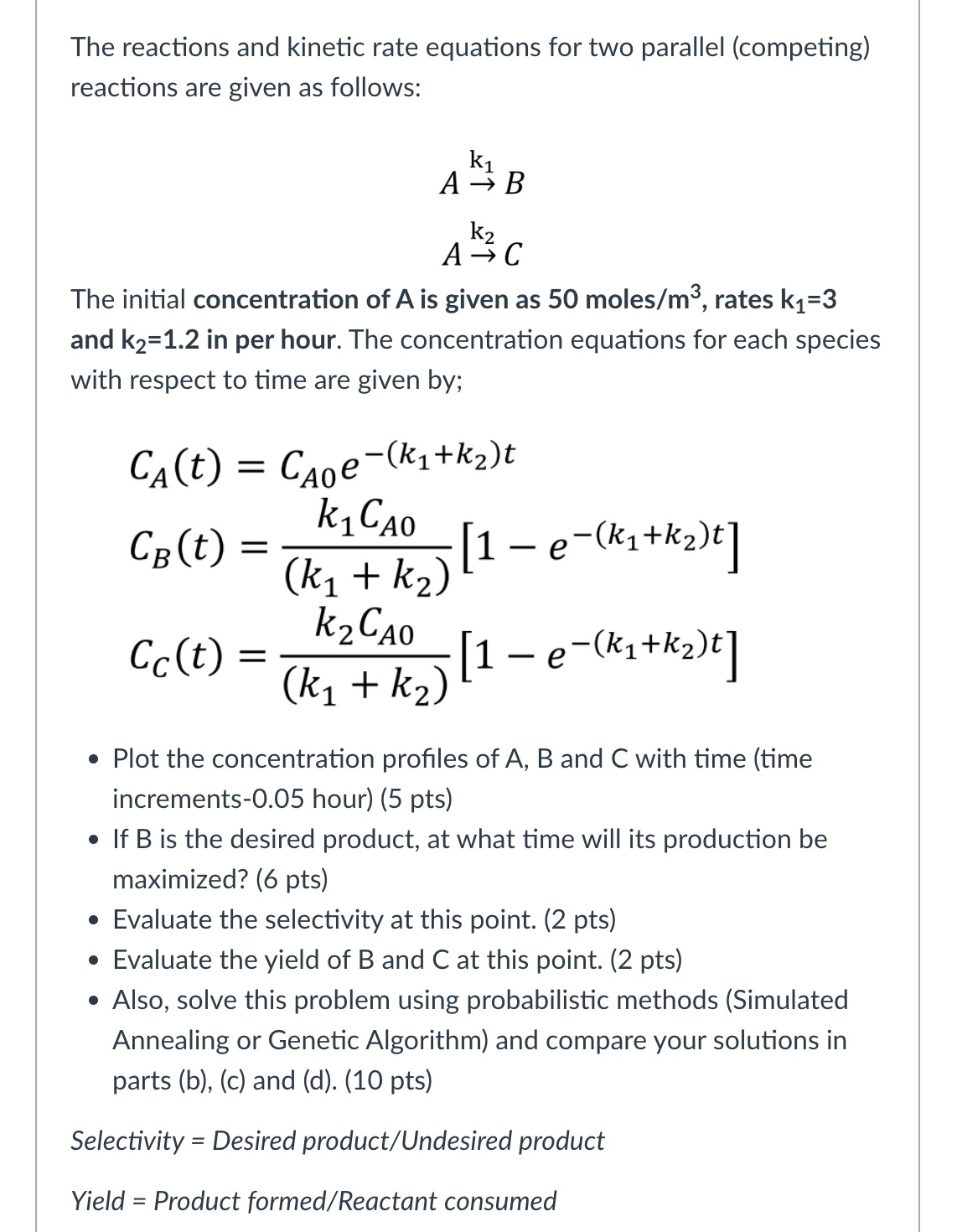
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


