Question: Gibbs adsorption equation (15 points) The table below shows the measured surface tension of aq. solution of surfactant dodecyldimethylammonium chloride from (a) no added salt
Gibbs adsorption equation (15 points) The table below shows the measured surface tension of aq. solution of surfactant dodecyldimethylammonium chloride from (a) no added salt and (b) 0.20 M NaCl solution. Assume 20oC temperature
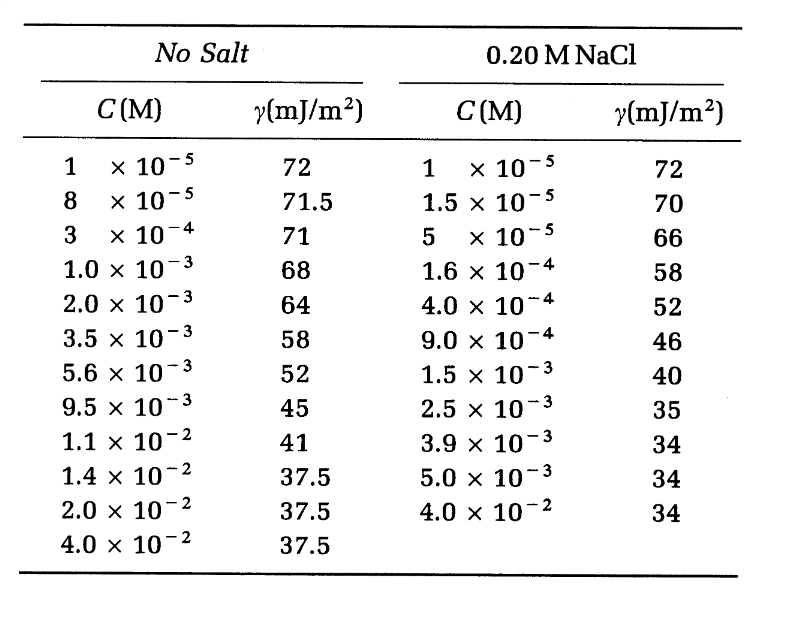
Estimate the surface excesses and areas/molecule at c1 = 0.001 M and c2 = 0.014 M in both (a) and (b) cases. Discuss the difference between no salt and NaCl results. Explain what happens at high concentrations?
No Salt 0.20 M NaCl C(M) y(mJ/m2) C(M) y(mJ/m) 1 8 x 10-5 o x 10-5 x 10-5 X 10-4 72 71.5 71 68 72 70 66 3 x 10-5 1.0 x 107 2.0 x 10-3 3.5 x 10-3 5.6 x 10-3 9.5 x 10? 1.1 x 10-2 1.4 x 10-2 2.0 x 10-2 4.0 x 10-2 1 1.5 x 10-5 5 1.6 x 10-4 4.0 x 10-4 9.0 x 10-4 1.5 x 10-3 2.5 x 10-3 3.9 X 10-3 5.0 x 10-3 4.0 x 10-2 64 58 52 45 41 37.5 37.5 37.5 - 58 52 46 40 35 34 34 34
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


