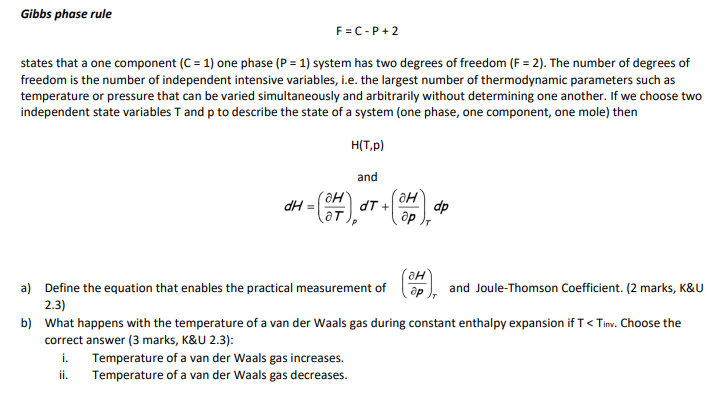
Question: Gibbs phase rule F = C - P + 2 states that a one component ( C = 1 ) one phase ( P =
Gibbs phase rule
states that a one component one phase system has two degrees of freedom The number of degrees of
freedom is the number of independent intensive variables, ie the largest number of thermodynamic parameters such as
temperature or pressure that can be varied simultaneously and arbitrarily without determining one another. If we choose two
independent state variables and to describe the state of a system one phase, one component, one mole then
and
a Define the equation that enables the practical measurement of and JouleThomson Coefficient. marks, K&U
b What happens with the temperature of a van der Waals gas during constant enthalpy expansion if Choose the
correct answer marks, &:
Temperature a van der Waals gas increases.
Temperature a van der Waals gas decreases.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


