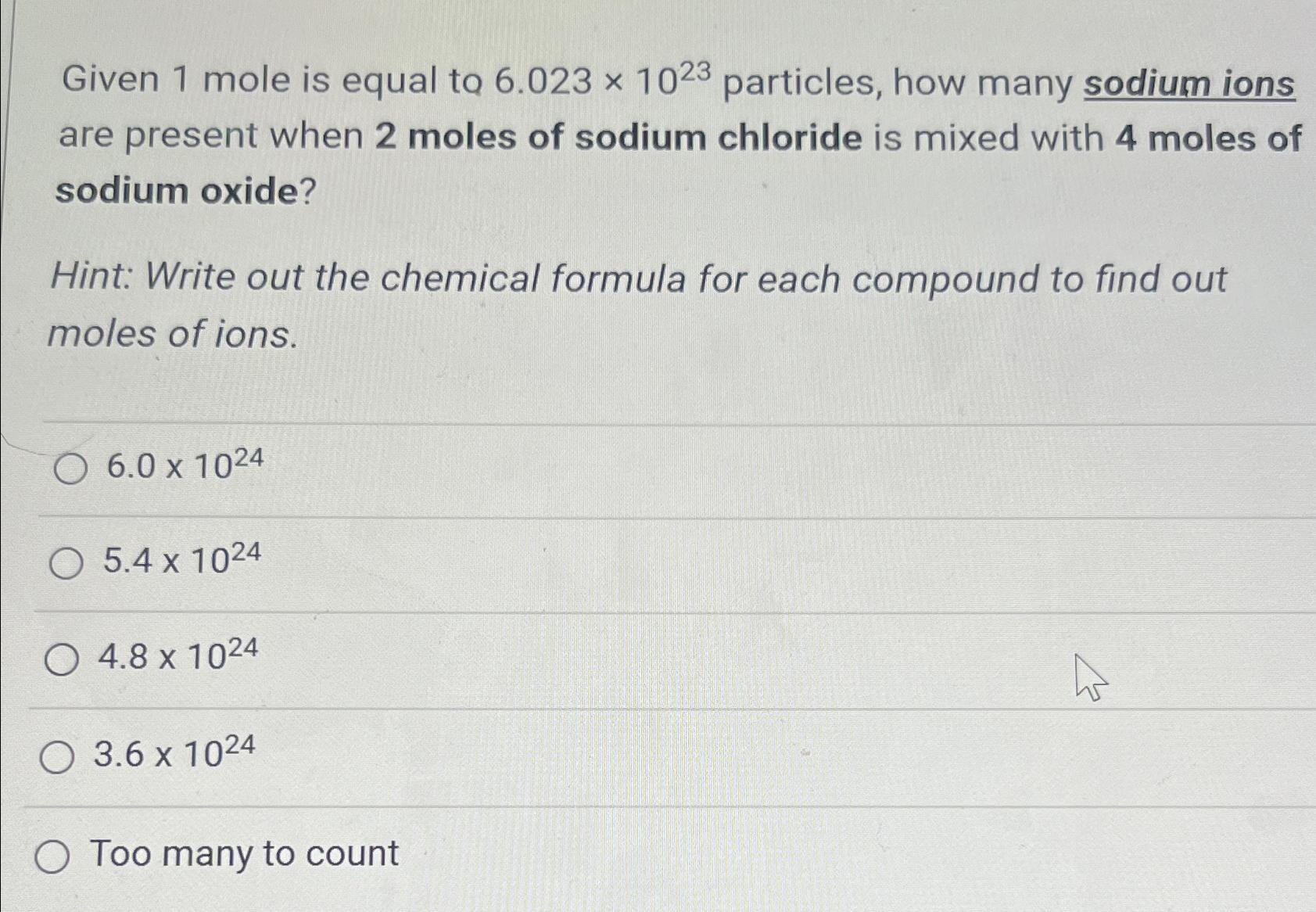
Question: Given 1 mole is equal to 6 . 0 2 3 1 0 2 3 particles, how many sodium ions are present when 2 moles
Given mole is equal to particles, how many sodium ions are present when moles of sodium chloride is mixed with moles of sodium oxide?
Hint: Write out the chemical formula for each compound to find out moles of ions.
Too many to count
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


