Question: Given aqueous reaction A -> R+S with kinetic study result as below Time, h 0 36 65 100 160 OC Total pressure, at 0.1823 0.1453
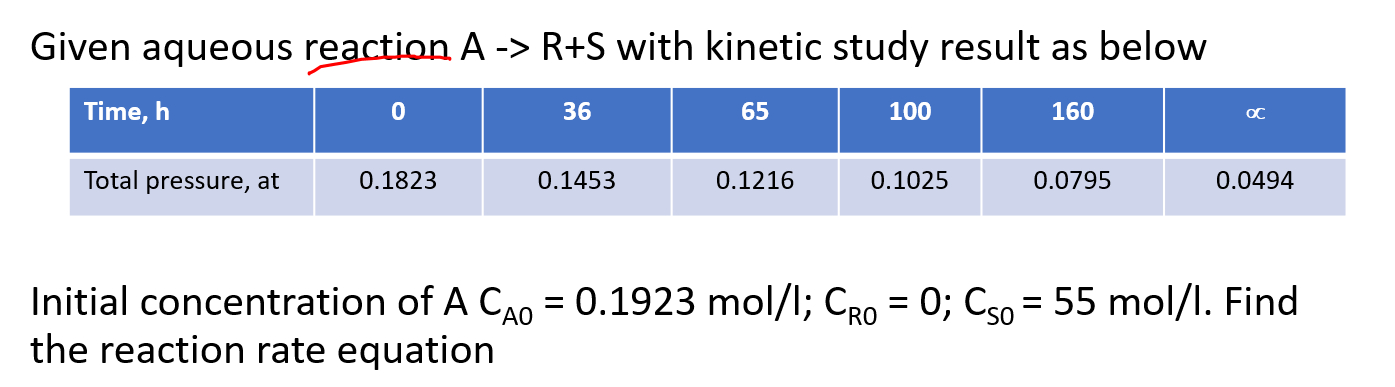
Given aqueous reaction A -> R+S with kinetic study result as below Time, h 0 36 65 100 160 OC Total pressure, at 0.1823 0.1453 0.1216 0.1025 0.0795 0.0494 = = Initial concentration of A Cao = 0.1923 mol/l; Cro = 0; Cso = 55 mol/l. Find the reaction rate equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


