Question: Compare the slope value determined from the hand-drawn graph with the slope from the computer-generated graph. How similar or different are the density values? Why
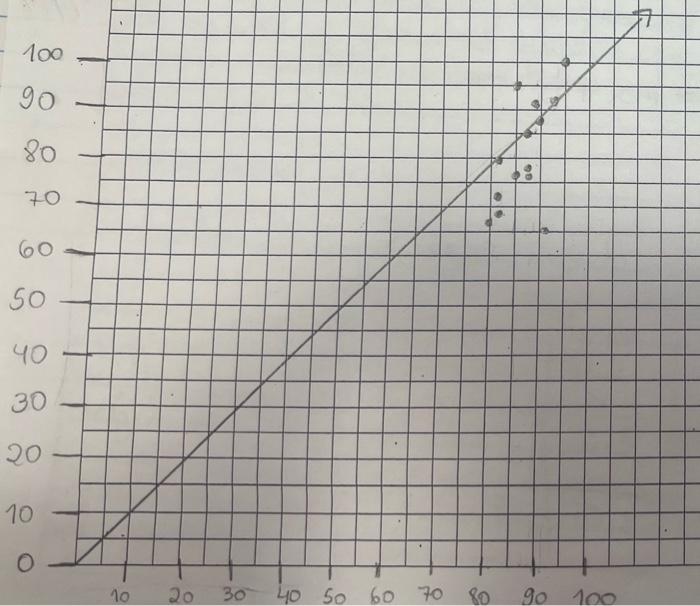
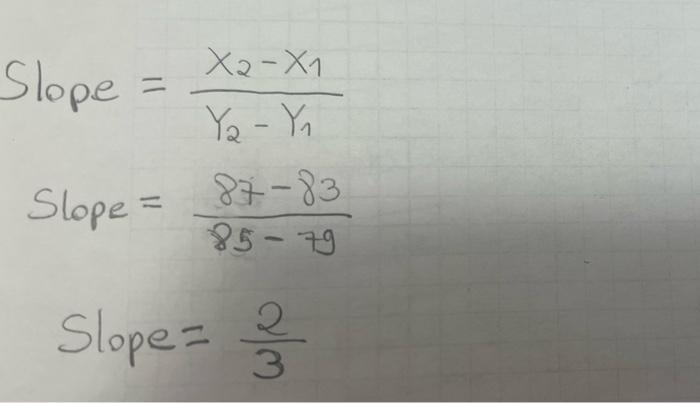
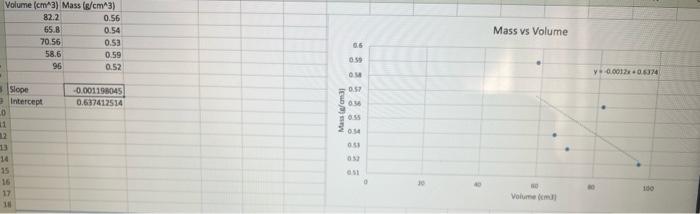
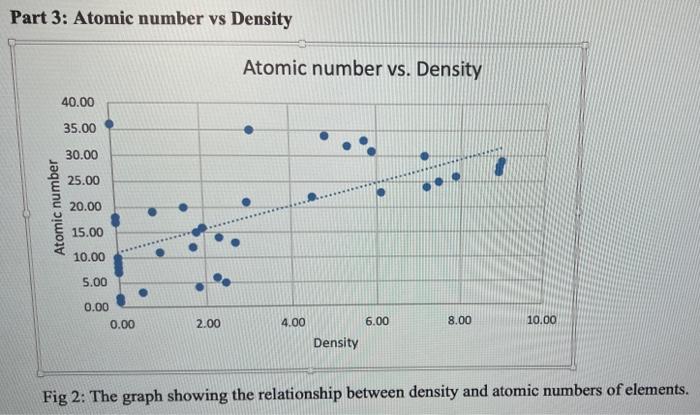
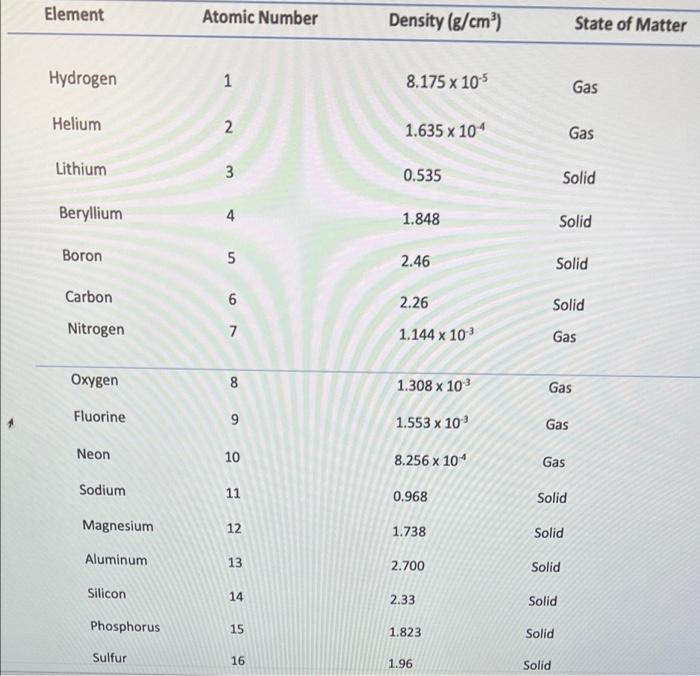
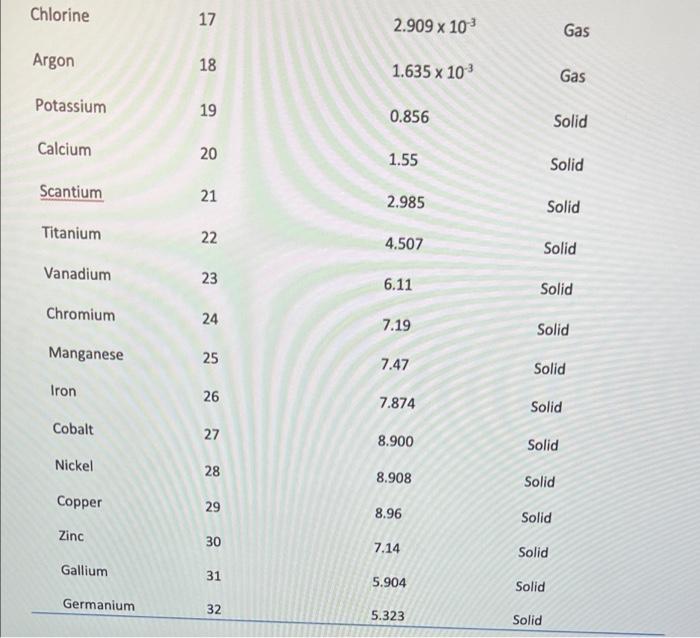

- 100 90 80 70 19 60 so 40 30 10 10 20 30 40 50 60 60 70 70 80 90 100 Slope = Xa-X1 Yo - Y, 87-83 85 - 79 Slope = 2 Slopes 3 Volume (cm3) Mass (g/cm3) 82.2 0.56 65.B 0.54 70.56 0.53 58.6 0.59 96 0.52 Mass vs Volume 0:59 OM y000130514 0:57 -0.001198045 0.637412514 Mass Comas 0:14 5 Slope Intercept 0 1 12 13 14 15 16 17 05 100 Volume Part 3: Atomic number vs Density Atomic number vs. Density 40.00 35.00 30.00 25.00 Atomic number 20.00 15.00 10.00 5.00 0.00 0.00 2.00 4.00 6.00 8.00 10.00 Density Fig 2: The graph showing the relationship between density and atomic numbers of elements. Element Atomic Number Density (g/cm) State of Matter Hydrogen 1 8.175 x 105 Gas Helium 2 1.635 10" Gas Lithium 3 0.535 Solid Beryllium 4 1.848 Solid Boron 5 2.46 Solid Carbon 6 Solid Nitrogen 2.26 1.144 x 10 7 Gas Oxygen 8 1.308 x 10 Gas Fluorine 9 1.553 x 10 Gas Neon 10 8.256 x 10 Gas Sodium 11 0.968 Solid Magnesium 12 1.738 Solid Aluminum 13 2.700 Solid Silicon 14 2.33 Solid Phosphorus 15 1.823 Solid Sulfur 16 1.96 Solid Chlorine 2.909 x 10 Gas 17 18 Argon 1.635 x 10 Gas Potassium 19 0.856 Solid Calcium 20 1.55 Solid Scantium 21 2.985 Solid Titanium 22 4.507 Solid Vanadium 23 6.11 Solid Chromium 24 7.19 Solid Manganese 25 7.47 Solid Iron 26 7.874 Solid Cobalt 27 8.900 Solid Nickel 28 8.908 Solid Copper 29 8.96 Solid Zinc 30 7.14 Solid Gallium 31 5.904 Solid Germanium 32 5.323 Solid Arsenic 33 5.727 Solid Selenium 34 4.819 Solid Bromine 35 3.12 Liquid Krypton 36 3.425 x 10 Gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


