Question: Given; E G = -79.6127 Hartree, E T = -79.60806 Hartree, K B = The Boltzmann constant (1.3806x10 -23 J/K), and T= 300 K, what
Given; EG= -79.6127 Hartree, ET= -79.60806 Hartree, KB= The Boltzmann constant (1.3806x10-23J/K), and T= 300 K, what is the Boltzmann factor?
Use 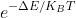
What have I done wrong here? The answer should be closer to 1 since the probability of ethane rotating around its bond is relatively high.

e- -/ H 41 -3.47099 T Ethane (should be close to 14 Ly easier to rotate around a single bond Eg where j 67 - -3.47078 J Ke = 1.380681023/12 ground transition -(Ex Ey)/ Kit e TE 300k - 71.6127 Hortice 2.09023 x105]my. 6.022x10-23 mo?" = -3.47097810305 -7%. Goda Horse = -2.0901x1064 6.022x10-23 =-3. 470 78X10 30 J more -(-3 4708 no)+(-3440x1039) (1.3606x10* /A3ook) - 20211075/4.14*10-215 e Fe .-4.88x1046 wo * This should be closer to 1 *
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


