Question: Given that x1 = 0.11, x2 = 0.33, x3 = 0.55, x4 = 0.77. x5 = 0.99 100 moles of a mixture containing A and
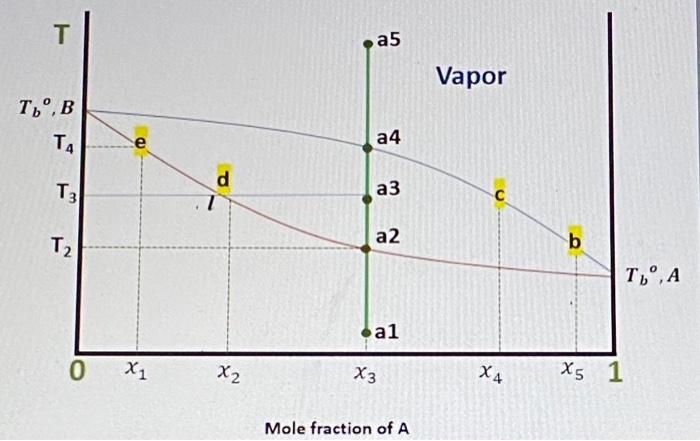
T a5 Vapor , TA D a4 d 7 T2 a2 b T', A .al 0 X1 X2 X3 X4 X5 1 Mole fraction of A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


