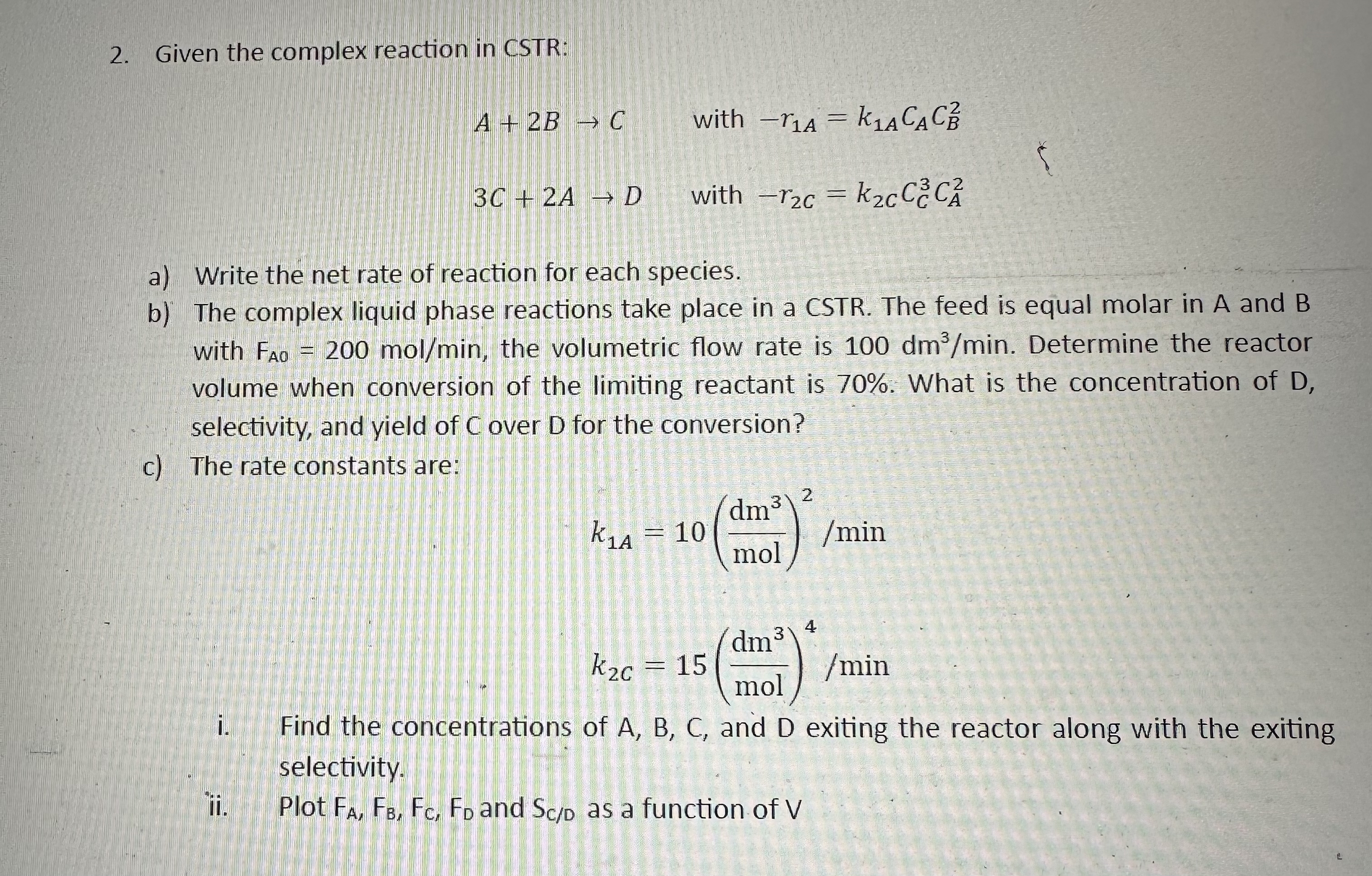
Question: Given the complex reaction in CSTR: A + 2 B C with - r 1 A = k 1 A C A C B 2
Given the complex reaction in CSTR:
with
with
a Write the net rate of reaction for each species.
b The complex liquid phase reactions take place in a CSTR The feed is equal molar in A and with the volumetric flow rate is Determine the reactor volume when conversion of the limiting reactant is What is the concentration of selectivity, and yield of C over D for the conversion?
c The rate constants are:
i Find the concentrations of and exiting the reactor along with the exiting selectivity.
ii Plot and as a function of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


