Question: Given the data below, please show me step by step how I can use the formulas to fill in the blanks. Thanks The final temperature,
Given the data below, please show me step by step how I can use the formulas to fill in the blanks. Thanks
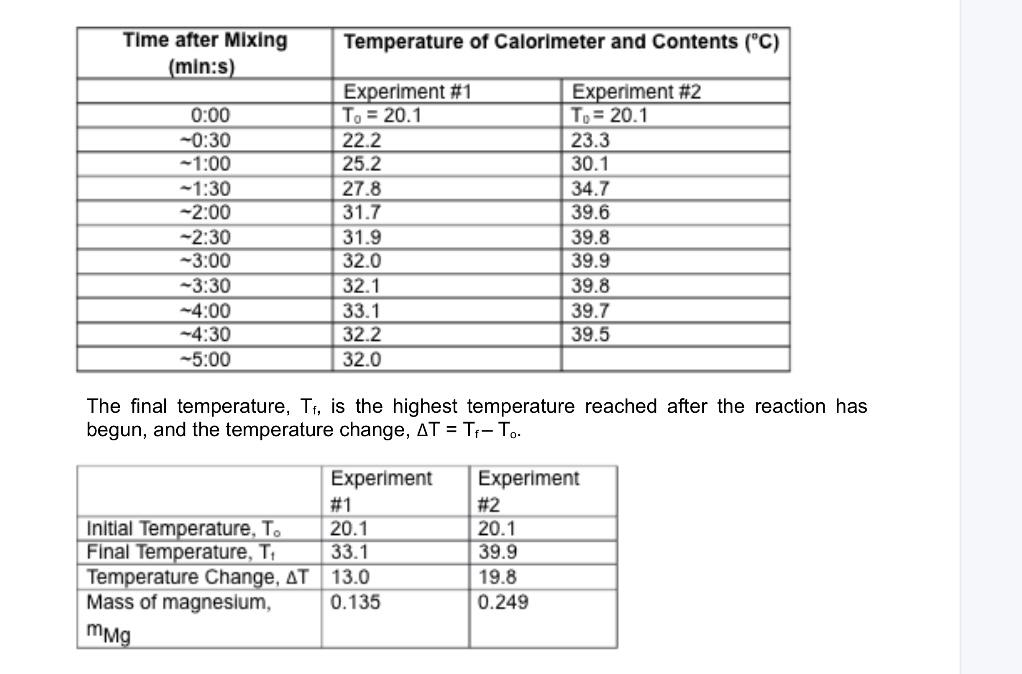

The final temperature, Tf, is the highest temperature reached after the reaction has begun, and the temperature change, T=TfTo. Calculation of Molar Heat of Reaction The heat, Q, absorbed by the calorimeter and its contents during the course of the reaction can be calculated from the change in temperature, T, using equation 1 . Q=CanT+mCvT Eq. 1 Here Cal is the heat capacity of the Styrofoam calorimeter, m is the mass of the calorimeter contents, and Cp is the specific heat of those contents. You may take Caa to equal 20.0J/C. Since the contents are mostly water, which has a density of 1.0g/mL, the mass of the contents, m, can be approximated as 50.0g. You may also take the specifie heat, C5, of the solution to be that of pure water (4.184J/gC). The heat of the reaction has the same magnitude as Q. but opposite sign. To calculate the molar heat of reaction, Ht, the heat of the reaction must be divided by the number of moles of Mg, nute. Hrn=nugQ Eq. 2 The number of moles of Mg, nMg, is calculated by dividing the mass of Mg, mMp0 by the molar mass of Mg (24.3g/mol): nmp=24,3g/molmc Eq. 3 Complete the following table. Show your calculations for Exp, 1 in the space below the table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


