Question: Given the data in the table below, AHxn for the reaction Ca(OH)2 + 2H2AsO4 Ca(H_AsO4)2 + 2H20 is kl. [A] -4219 [B] -130.4 [C] -4519
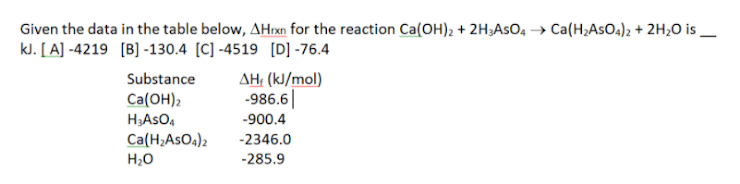
Given the data in the table below, AHxn for the reaction Ca(OH)2 + 2H2AsO4 Ca(H_AsO4)2 + 2H20 is kl. [A] -4219 [B] -130.4 [C] -4519 [D] -76.4 Substance AH (kJ/mol Ca(OH)2 -986.61 HASOA -900.4 Ca(H2AsO4)2 -2346.0 H2O -285.9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


