Question: Given the following connected electrodes (through a wire), a. Write the half-cell reactions. b. What is the balanced overall reaction? c. Calculate the Ecell (cell
Given the following connected electrodes (through a wire),
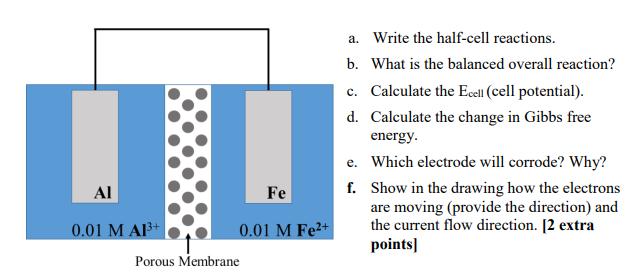
a. Write the half-cell reactions. b. What is the balanced overall reaction? c. Calculate the Ecell (cell potential). d. Calculate the change in Gibbs free energy. e. Which electrode will corrode? Why? f. Show in the drawing how the electrons are moving (provide the direction) and the current flow direction. [2 extra points]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


