Question: NOTE: use at least three non-zero decimal places in all calculations. Consider the following cell operating at 25C. Pt (s)|Ag+(0.005 M), Ag2+(0.0035 M)||Mno (0.10M), 1+(0.05
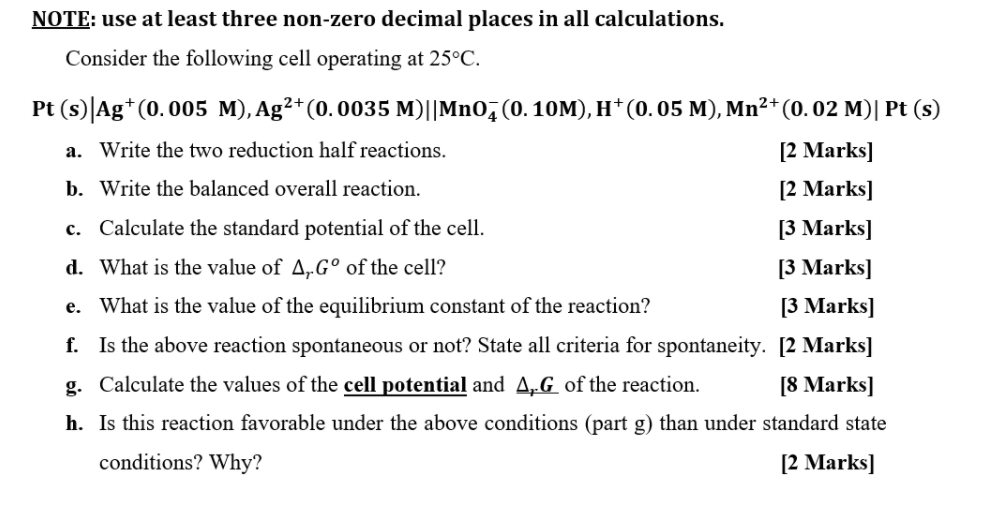
NOTE: use at least three non-zero decimal places in all calculations. Consider the following cell operating at 25C. Pt (s)|Ag+(0.005 M), Ag2+(0.0035 M)||Mno (0.10M), 1+(0.05 M), Mn2+(0.02 M)| Pt (s) a. Write the two reduction half reactions. [2 Marks] b. Write the balanced overall reaction. [2 Marks] c. Calculate the standard potential of the cell. [3 Marks] d. What is the value of 4, G of the cell? [3 Marks) e. What is the value of the equilibrium constant of the reaction? [3 Marks) f. Is the above reaction spontaneous or not? State all criteria for spontaneity. [2 Marks] g. Calculate the values of the cell potential and 4G of the reaction. [8 Marks] h. Is this reaction favorable under the above conditions (part g) than under standard state conditions? Why? [2 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


