Question: Given the following data, I need help with solving and understanding the last one. However, I am also quite unsure about my calculations for q
Given the following data, I need help with solving and understanding the last one. However, I am also quite unsure about my calculations for q water and delta H for the reaction. I would really appreciate it if I can receive some help verifying it and/or commenting on whether I am on the right track or not. Thank you!

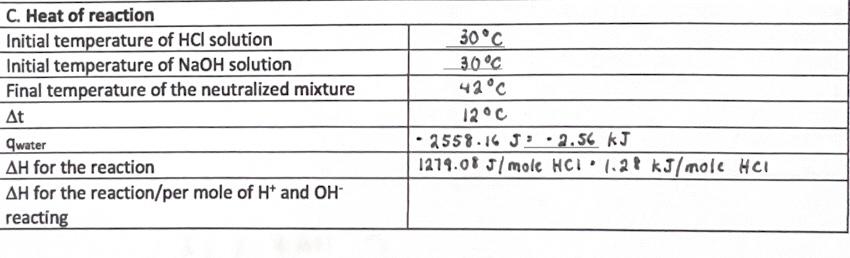
Heat of Reaction Rinse out your calorimeter with distilled water, pouring the rinse into the sink. In a graduated cylinder, measure out 25mL of 2.00MHCl; pour this into the calorimeter. Rinse out the cylinder with distilled water and measure out 25mL of 2.00MNaOH. Measure the temperature of the acid and of the base, making sure to rinse and dry the thermometer before dipping it into the solutions. Put the thermometer back into the calorimeter cover. Pour the NaOH solution into the HCl solution and cover the calorimeter. Stir the reaction mixture, and record the maximum temperature that is reached by the neutralized solution. Calculate the enthalpy of neutralization. Since the masses of the solutions were not determined, use a value of 1.02g/mL average density for both solutions in this experiment to calculate the mass of the solutions. Use 4.18J/gC for the specific heat of the solutions. C. Heat of reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


