Question: help quick!!! At high temperatures, NO2 decomposes to NO and O2 according to the following reaction: NO2(g) NO(g)+1/2O2(g) A graduate student measures the [NO2] vs.
help quick!!! 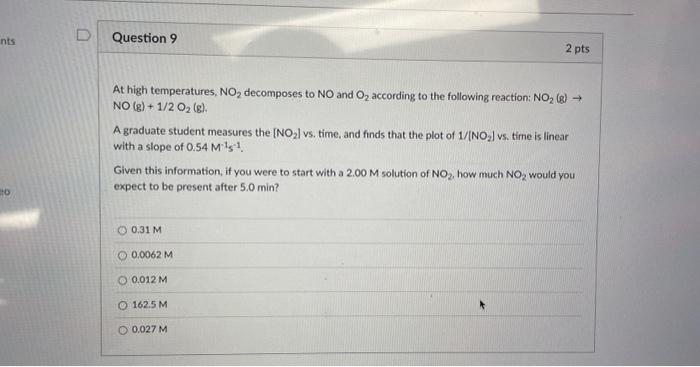

At high temperatures, NO2 decomposes to NO and O2 according to the following reaction: NO2(g) NO(g)+1/2O2(g) A graduate student measures the [NO2] vs. time, and finds that the plot of 1/[NO2] vs. time is linear with a slope of 0.54M1s1. Given this information, if you were to start with a 2.00M solution of NO2, how much NO2 would you expect to be present after 5.0min ? 0.31M0.0062M0.012M162.5M0.027M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


