Question: Given the image below, which includes 25% conversion in reactor, 10% purge and recycle stream. Conduct a mass balance producing 100 tons of ammonia per
Given the image below, which includes 25% conversion in reactor, 10% purge and recycle stream. Conduct a mass balance producing 100 tons of ammonia per day (assume the whole calculation done is one day). Consider the chemical reaction occuring in the reactor.
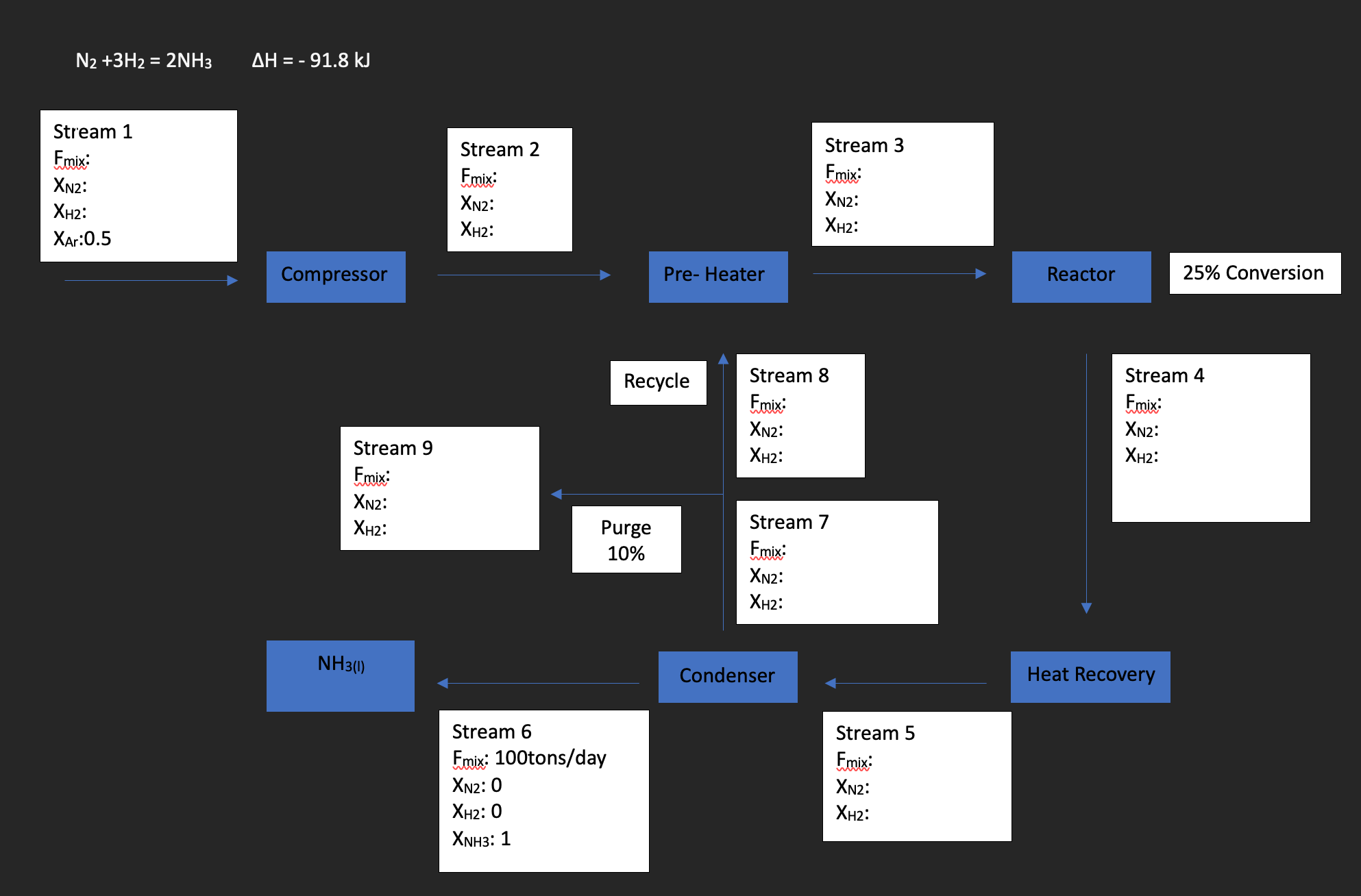
N2+3H2=2NH3H=91.8kJ Stream 1 Fmix: XN2 : XH2 : XAr:0.5 Stream 2 Fmix: miv XN2 : XH2 : Stream 3 Fmix: XN2 : XH2 : Compressor Stream 9 Fmix: XN2 : XH2 : Purge 10% Stream 8 XN2 : XH2 : NH3(1) Condenser Stream 6 Fmix::100 tons/day XN2:0 XH2:0 XNH3:1 Stream 4 Fmix: XN2 : XH2 : 25% Conversion Stream 7 XN2 : XH2 : Heat Recovery Stream 5 XN2 : XH2 : N2+3H2=2NH3H=91.8kJ Stream 1 Fmix: XN2 : XH2 : XAr:0.5 Stream 2 Fmix: miv XN2 : XH2 : Stream 3 Fmix: XN2 : XH2 : Compressor Stream 9 Fmix: XN2 : XH2 : Purge 10% Stream 8 XN2 : XH2 : NH3(1) Condenser Stream 6 Fmix::100 tons/day XN2:0 XH2:0 XNH3:1 Stream 4 Fmix: XN2 : XH2 : 25% Conversion Stream 7 XN2 : XH2 : Heat Recovery Stream 5 XN2 : XH2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


