Question: Given the lead-tin phase diagram as shown below, write down the eutectic reaction at the eutectic point with compositions for each phase specified and Describe
Given the lead-tin phase diagram as shown below, write down the eutectic reaction at the eutectic point with compositions for each phase specified and Describe in brief why such a microstructure is preferred?
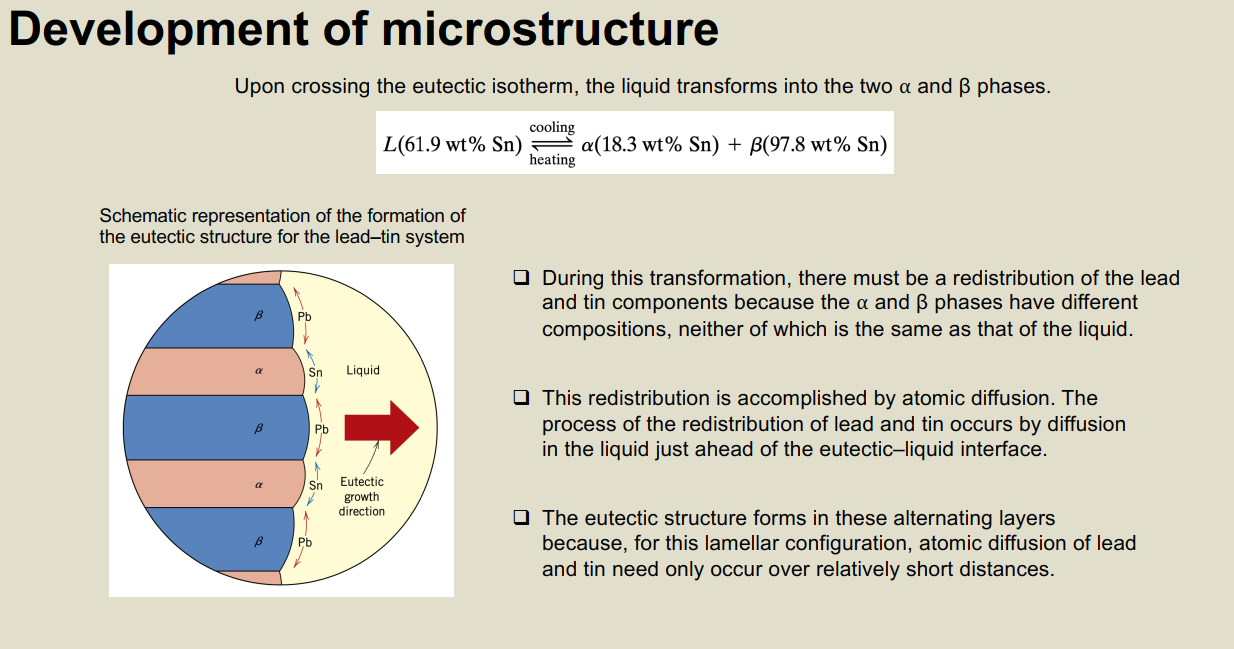
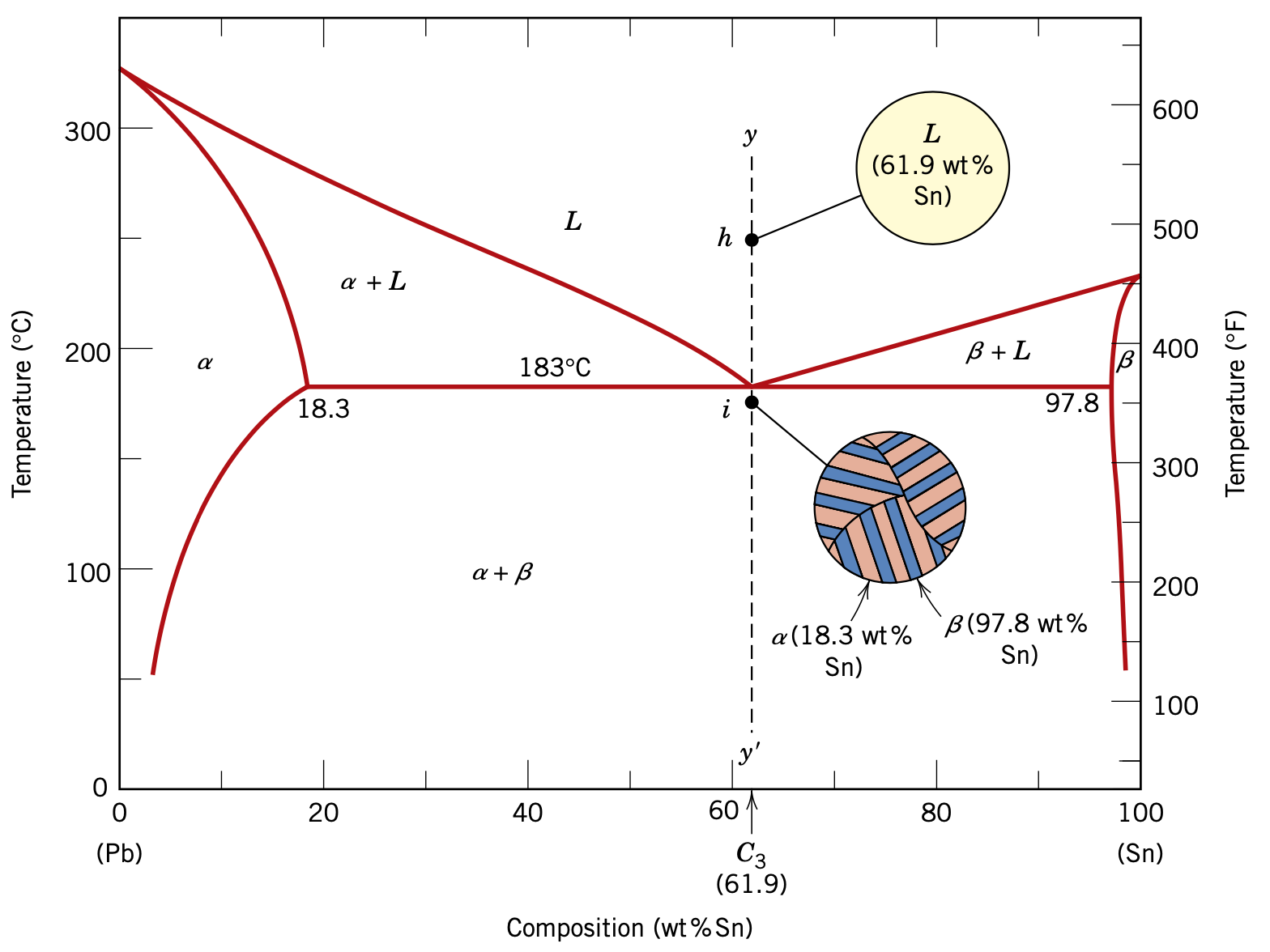
Development of microstructure Upon crossing the eutectic isotherm, the liquid transforms into the two a and phases. cooling heating L(61.9 wt% Sn) a(18.3 wt% Sn) + (97.8 wt% Sn) Schematic representation of the formation of the eutectic structure for the lead-tin system Sn Liquid B Pb R a Sn Eutectic growth direction During this transformation, there must be a redistribution of the lead and tin components because the a and phases have different compositions, neither of which is the same as that of the liquid. This redistribution is accomplished by atomic diffusion. The process of the redistribution of lead and tin occurs by diffusion in the liquid just ahead of the eutectic-liquid interface. The eutectic structure forms in these alternating layers because, for this lamellar configuration, atomic diffusion of lead and tin need only occur over relatively short distances.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


