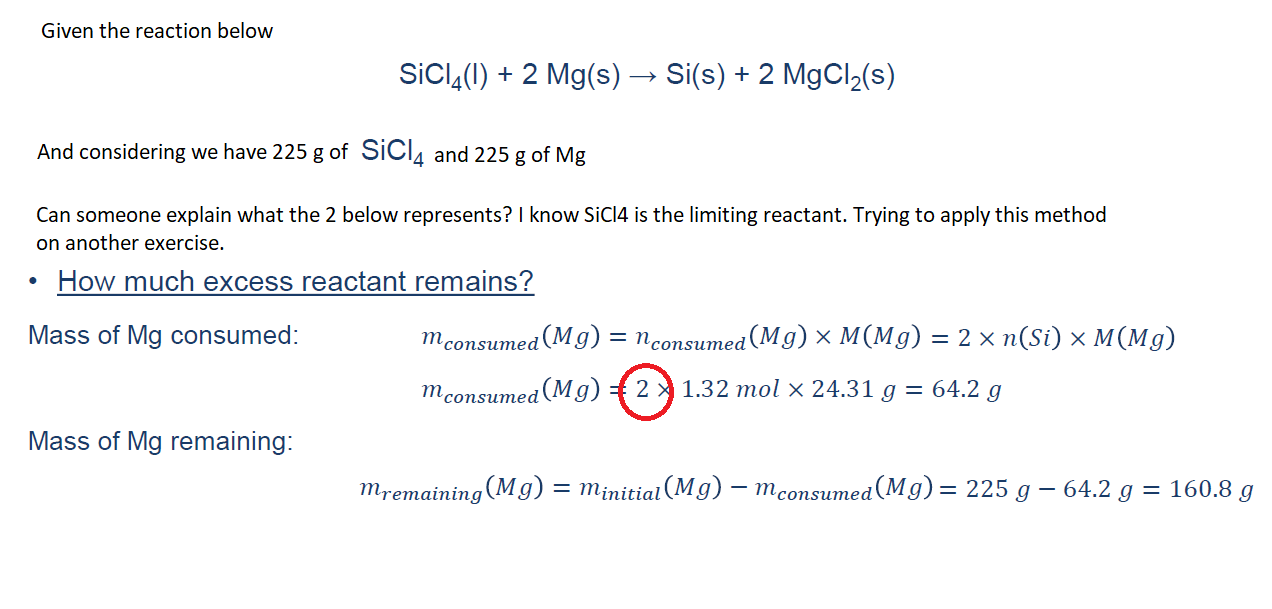
Question: Given the reaction below S i C l 4 ( l ) + 2 M g ( s ) S i ( s ) +
Given the reaction below
And considering we have of and of
Can someone explain what the below represents? I know SiCl is the limiting reactant. Trying to apply this method
on another exercise.
How much excess reactant remains?
Mass of Mg consumed:
mol
Mass of remaining:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


