Question: Given the same reactant concentrations, the below reaction at 227 , C is 21.1 times as fast as the same reaction at 167 ,
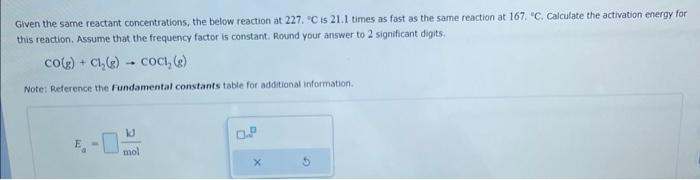
Given the same reactant concentrations, the below reaction at 227 , " C is 21.1 times as fast as the same reaction at 167 , " C. Calculate the activation energy for this reaction. Assume that the frequency factor is constant. Round your answer to 2 significant digits. CO(g)+Cl2(g)COCl2(g) Note: Reference the fundamental constants table for additional information. Ea=molkd
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


