Question: Given the sample titration data from a titration of a prepared sodium hydroxide (NaOH) solution with potassium hydrogen phthalate (KHP) (Eq. 1), fill in the
Given the sample titration data from a titration of a prepared sodium hydroxide (NaOH) solution with potassium hydrogen phthalate (KHP) (Eq. 1), fill in the empty data columns. The molar mass of KHP is 204.22 g/mol.
NaOH (aq) + KHC8H4O4 (aq) KNaC8H4O4 (aq) + H2O (l) (Equation 1)
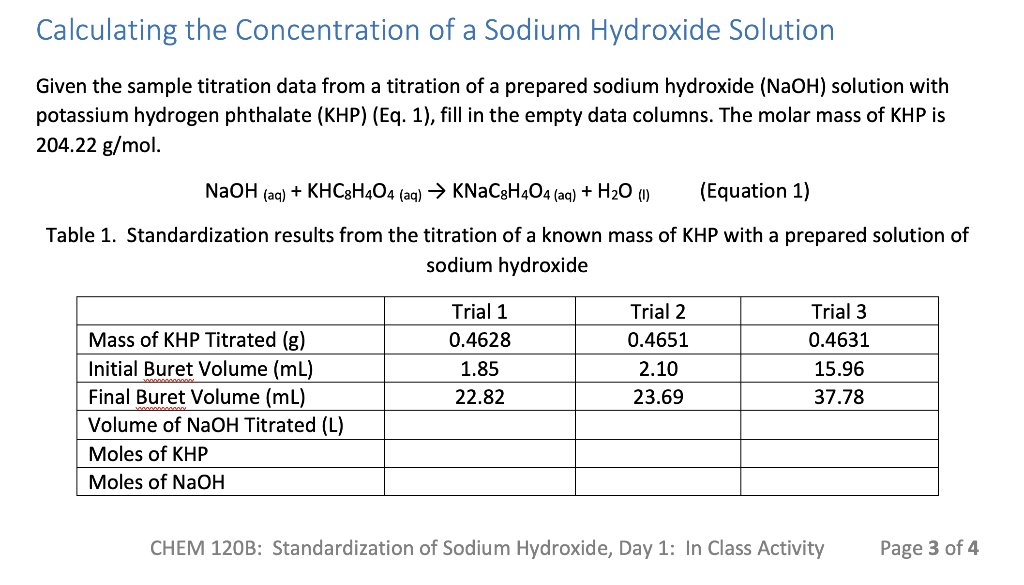
Calculating the Concentration of a Sodium Hydroxide Solution Given the sample titration data from a titration of a prepared sodium hydroxide (NaOH) solution with potassium hydrogen phthalate (KHP) (Eq. 1), fill in the empty data columns. The molar mass of KHP is 204.22 g/mol. NaOH (aq) + KHC3H404 (aq) KNaC3H404 (aq) + H20 (1) (Equation 1) Table 1. Standardization results from the titration of a known mass of KHP with a prepared solution of sodium hydroxide Trial 1 0.4628 1.85 22.82 Trial 2 0.4651 2.10 23.69 Trial 3 0.4631 15.96 37.78 Mass of KHP Titrated (g) Initial Buret Volume (mL) Final Buret Volume (mL) Volume of NaOH Titrated (L) Moles of KHP Moles of NaOH CHEM 120B: Standardization of Sodium Hydroxide, Day 1: In Class Activity Page 3 of 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


