Question: Given the standard enthalpy changes for the following two reactions: (1) N2(g)+2O2(g)N2O4(g)H=9.2kJ (2) 2N2O(g)2N2(g)+O2(g)H=164.2kJ What is the standard enthalpy change for the following reaction? (3)
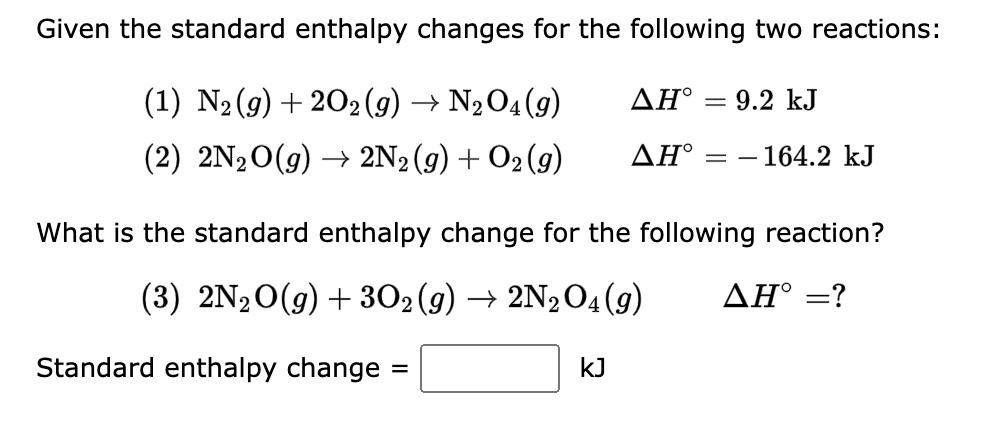
Given the standard enthalpy changes for the following two reactions: (1) N2(g)+2O2(g)N2O4(g)H=9.2kJ (2) 2N2O(g)2N2(g)+O2(g)H=164.2kJ What is the standard enthalpy change for the following reaction? (3) 2N2O(g)+3O2(g)2N2O4(g)H= ? Standard enthalpy change =kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


