Question: The data in the table below were obtained for the reaction: 2H2(g)+2NO(g)N2(g)+2H2O(g) The rate constant, k, for this reaction is 103M2s1. Please record your answer
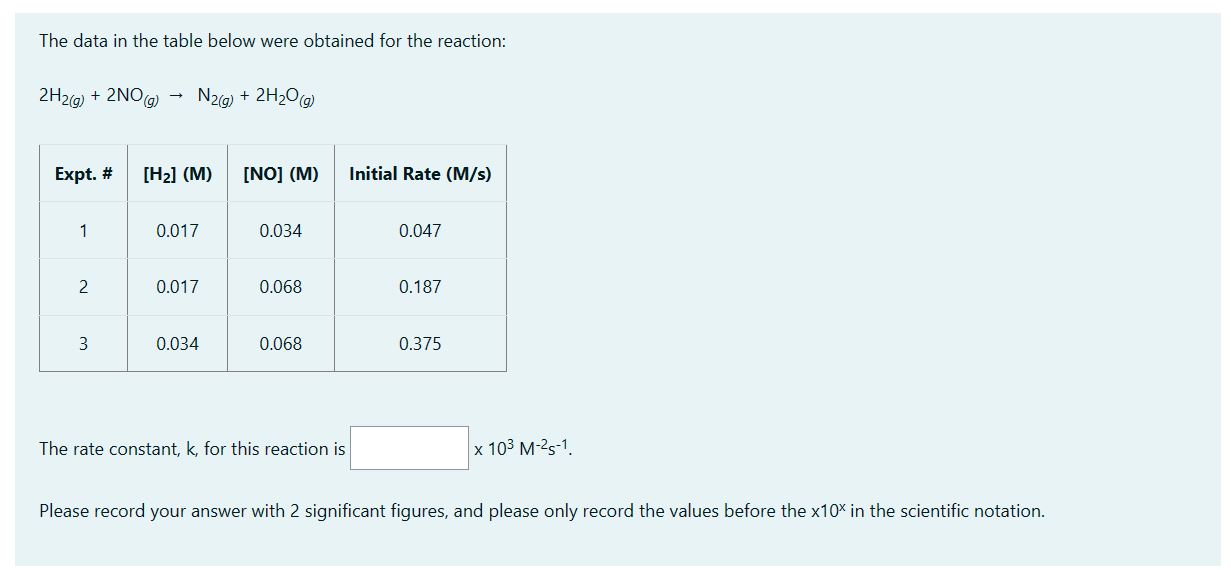
The data in the table below were obtained for the reaction: 2H2(g)+2NO(g)N2(g)+2H2O(g) The rate constant, k, for this reaction is 103M2s1. Please record your answer with 2 significant figures, and please only record the values before the x10x in the scientific notation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


