Question: Given these standard reduction potentials at 25 C 3+ 1. Cr+ + e Cr+ 2. Cr+ + 2e 3+ Cr+ + 3e O E
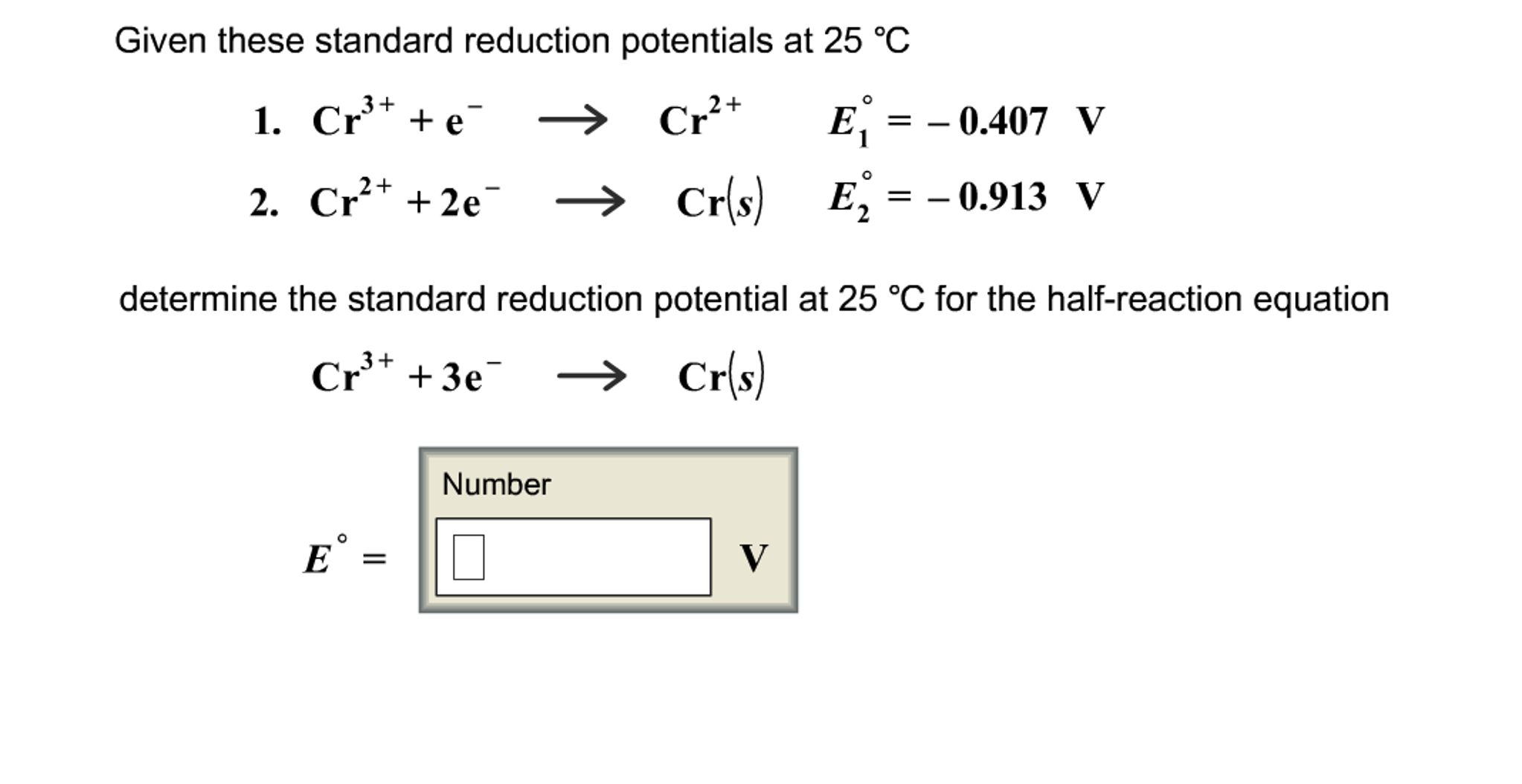
Given these standard reduction potentials at 25 C 3+ 1. Cr+ + e Cr+ 2. Cr+ + 2e 3+ Cr+ + 3e O E = Cr(s) determine the standard reduction potential at 25 C for the half-reaction equation Cr(s) Number O E = E = -0.913 V V = - 0.407 V
Step by Step Solution
3.50 Rating (163 Votes )
There are 3 Steps involved in it
The given reactions are as follows eCr 1 Cr 2 Cr 2e Crs 3 Cr 3eCr... View full answer

Get step-by-step solutions from verified subject matter experts


